Abstract
Juvenile idiopathic arthritis (JIA) is a chronic inflammation of joints in pediatric patients. Assessment of JIA disease activity is very difficult, because children cannot definitely describe their pain by themselves due to development of cognitive function during the pediatric period. Assessment of JIA disease activity is useful for quantitative measurement of patient status, monitoring therapeutic response, and disease course over time. This article reviewed objective assessment tool for JIA disease activity and described differences in assessment between adult rheumatoid arthritis and JIA.
Go to : 
REFERENCES
3. van Dijkhuizen EH, Wulffraat NM. Early predictors of prognosis in juvenile idiopathic arthritis: a systematic literature review. Ann Rheum Dis. 2014; pii: annrheum-dis-2014-205265. [Epub ahead of print].

4. Luca NJ, Feldman BM. Disease activity measures in paediatric rheumatic diseases. Int J Rheumatol. 2013; 2013; 715352.

5. April KT, Feldman DE, Platt RW, Duffy CM. Comparison between Children with Juvenile Idiopathic Arthritis (JIA) and their parents concerning perceived Quality of Life. Qual Life Res. 2006; 15:655–61.

6. Carle AC, Dewitt EM, Seid M. Measures of health status and quality of life in juvenile rheumatoid arthritis: Pediatric Quality of Life Inventory (PedsQL) Rheumatology Module 3.0, Juvenile Arthritis Quality of Life Questionnaire (JAQQ), Paediatric Rheumatology Quality of Life Scale (PRQL), and Childhood Arthritis Health Profile (CAHP). Arthritis Care Res (Hoboken). 2011; 63(Suppl 11):S438–45.
7. Feldman BM, Grundland B, McCullough L, Wright V. Distinction of quality of life, health related quality of life, and health status in children referred for rheumatologic care. J Rheumatol. 2000; 27:226–33.
8. Dempster H, Porepa M, Young N, Feldman BM. The clinical meaning of functional outcome scores in children with juvenile arthritis. Arthritis Rheum. 2001; 44:1768–74.

9. Brunner HI, Ravelli A. Developing outcome measures for paediatric rheumatic diseases. Best Pract Res Clin Rheumatol. 2009; 23:609–24.

10. Rider LG, Giannini EH, Brunner HI, Ruperto N, James-Newton L, Reed AM, et al. International Myositis Assessment and Clinical Studies Group. International consensus on preliminary definitions of improvement in adult and juvenile myositis. Arthritis Rheum. 2004; 50:2281–90.

11. Burgos-Vargas R. Assessment of quality of life in children with rheumatic disease. J Rheumatol. 1999; 26:1432–5.
12. Ruperto N, Meiorin S, Iusan SM, Ravelli A, Pistorio A, Martini A. Consensus procedures and their role in pediatric rheumatology. Curr Rheumatol Rep. 2008; 10:142–6.

13. Giannini EH, Ruperto N, Ravelli A, Lovell DJ, Felson DT, Martini A. Preliminary definition of improvement in juvenile arthritis. Arthritis Rheum. 1997; 40:1202–9.

14. Seid M, Huang B, Niehaus S, Brunner HI, Lovell DJ. Determinants of health-related quality of life in children newly diagnosed with juvenile idiopathic arthritis. Arthritis Care Res (Hoboken). 2014; 66:263–9.

15. Ruperto N, Pistorio A, Ravelli A, Hasija R, Guseinova D, Filocamo G, et al. Paediatric Rheumatology International Trials Organisation (PRINTO). Criteria to define response to therapy in paediatric rheumatic diseases. Eur J Clin Pharmacol. 2011; 67(Suppl 1):125–31.

16. Giannini EH, Ilowite NT, Lovell DJ, Wallace CA, Rabinovich CE, Reiff A, et al. Pediatric Rheumatology Collaborative Study Group. Long-term safety and effectiveness of etanercept in children with selected categories of juvenile idiopathic arthritis. Arthritis Rheum. 2009; 60:2794–804.

17. Ruperto N, Ravelli A, Pistorio A, Malattia C, Cavuto S, Gado-West L, et al. Paediatric Rheumatology International Trials Organisation. Cross-cultural adaptation and psychometric evaluation of the Childhood Health Assessment Questionnaire (CHAQ) and the Child Health Questionnaire (CHQ) in 32 countries. Review of the general methodology. Clin Exp Rheumatol. 2001; 19(4 Suppl 23):S1–9.
18. Hochberg MC, Chang RW, Dwosh I, Lindsey S, Pincus T, Wolfe F. The American College of Rheumatology 1991 revised criteria for the classification of global functional status in rheumatoid arthritis. Arthritis Rheum. 1992; 35:498–502.

19. Ruperto N, Martini A. Network in pediatric rheumatology: the example of the Pediatric Rheumatology International Trials Organization. World J Pediatr. 2008; 4:186–91.

20. Wallace CA, Ruperto N, Giannini E. Childhood Arthritis and Rheumatology Research Alliance; Pediatric Rheumatology International Trials Organization; Pediatric Rheumatology Collaborative Study Group. Preliminary criteria for clinical remission for select categories of juvenile idiopathic arthritis. J Rheumatol. 2004; 31:2290–4.
21. Consolaro A, Bracciolini G, Ruperto N, Pistorio A, Magni-Manzoni S, Malattia C, et al. Paediatric Rheumatology International Trials Organization. Remission, minimal disease activity, and acceptable symptom state in juvenile idiopathic arthritis: defining criteria based on the juvenile arthritis disease activity score. Arthritis Rheum. 2012; 64:2366–74.

22. Singh G, Athreya BH, Fries JF, Goldsmith DP. Measurement of health status in children with juvenile rheumatoid arthritis. Arthritis Rheum. 1994; 37:1761–9.

23. Lovell DJ, Howe S, Shear E, Hartner S, McGirr G, Schulte M, et al. Development of a disability measurement tool for juvenile rheumatoid arthritis. The Juvenile Arthritis Functional Assessment Scale. Arthritis Rheum. 1989; 32:1390–5.

24. Howe S, Levinson J, Shear E, Hartner S, McGirr G, Schulte M, et al. Development of a disability measurement tool for juvenile rheumatoid arthritis. The Juvenile Arthritis Functional Assessment Report for Children and their Parents. Arthritis Rheum. 1991; 34:873–80.

25. Wright FV, Kimber JL, Law M, Goldsmith CH, Crombie V, Dent P. The Juvenile Arthritis Functional Status Index (JASI): a validation study. J Rheumatol. 1996; 23:1066–79.
26. Duffy CM, Arsenault L, Duffy KN, Paquin JD, Strawczynski H. The Juvenile Arthritis Quality of Life Questionnaire–development of a new responsive index for juvenile rheumatoid arthritis and juvenile spondyloarthritides. J Rheumatol. 1997; 24:738–46.
27. Duffy CM, Duffy KN. Health assessment in the rheumatic diseases of childhood. Curr Opin Rheumatol. 1997; 9:440–7.

28. Duffy CM. Measurement of health status, functional status, and quality of life in children with juvenile idiopathic arthritis: clinical science for the pediatrician. Rheum Dis Clin North Am. 2007; 33:389–402.

29. Varni JW, Seid M, Smith Knight T, Burwinkle T, Brown J, Szer IS. The PedsQL in pediatric rheumatology: reliability, validity, and responsiveness of the Pediatric Quality of Life Inventory Generic Core Scales and Rheumatology Module. Arthritis Rheum. 2002; 46:714–25.

30. Kook SH, Varni JW. Validation of the Korean version of the pediatric quality of life inventory 4.0 (PedsQL) generic core scales in school children and adolescents using the Rasch model. Health Qual Life Outcomes. 2008; 6:41.

31. Ramey DR, Raynauld JP, Fries JF. The health assessment questionnaire 1992: status and review. Arthritis Care Res. 1992; 5:119–29.

32. Meenan RF, Gertman PM, Mason JH. Measuring health status in arthritis. The arthritis impact measurement scales. Arthritis Rheum. 1980; 23:146–52.
33. van Riel PL. The development of the disease activity score (DAS) and the disease activity score using 28 joint counts (DAS28). Clin Exp Rheumatol. 2014; 32(5 Suppl 85):S65–74.
34. Consolaro A, Ruperto N, Bazso A, Pistorio A, Magni-Manzoni S, Filocamo G, et al. Paediatric Rheumatology International Trials Organisation. Development and validation of a composite disease activity score for juvenile idiopathic arthritis. Arthritis Rheum. 2009; 61:658–66.

35. Bazso A, Consolaro A, Ruperto N, Pistorio A, Viola S, Magni-Manzoni S, et al. Pediatric Rheumatology International Trials Organization. Development and testing of reduced joint counts in juvenile idiopathic arthritis. J Rheumatol. 2009; 36:183–90.

36. Merkel SI, Voepel-Lewis T, Shayevitz JR, Malviya S. The FLACC: a behavioral scale for scoring postoperative pain in young children. Pediatr Nurs. 1997; 23:293–7.
Go to : 
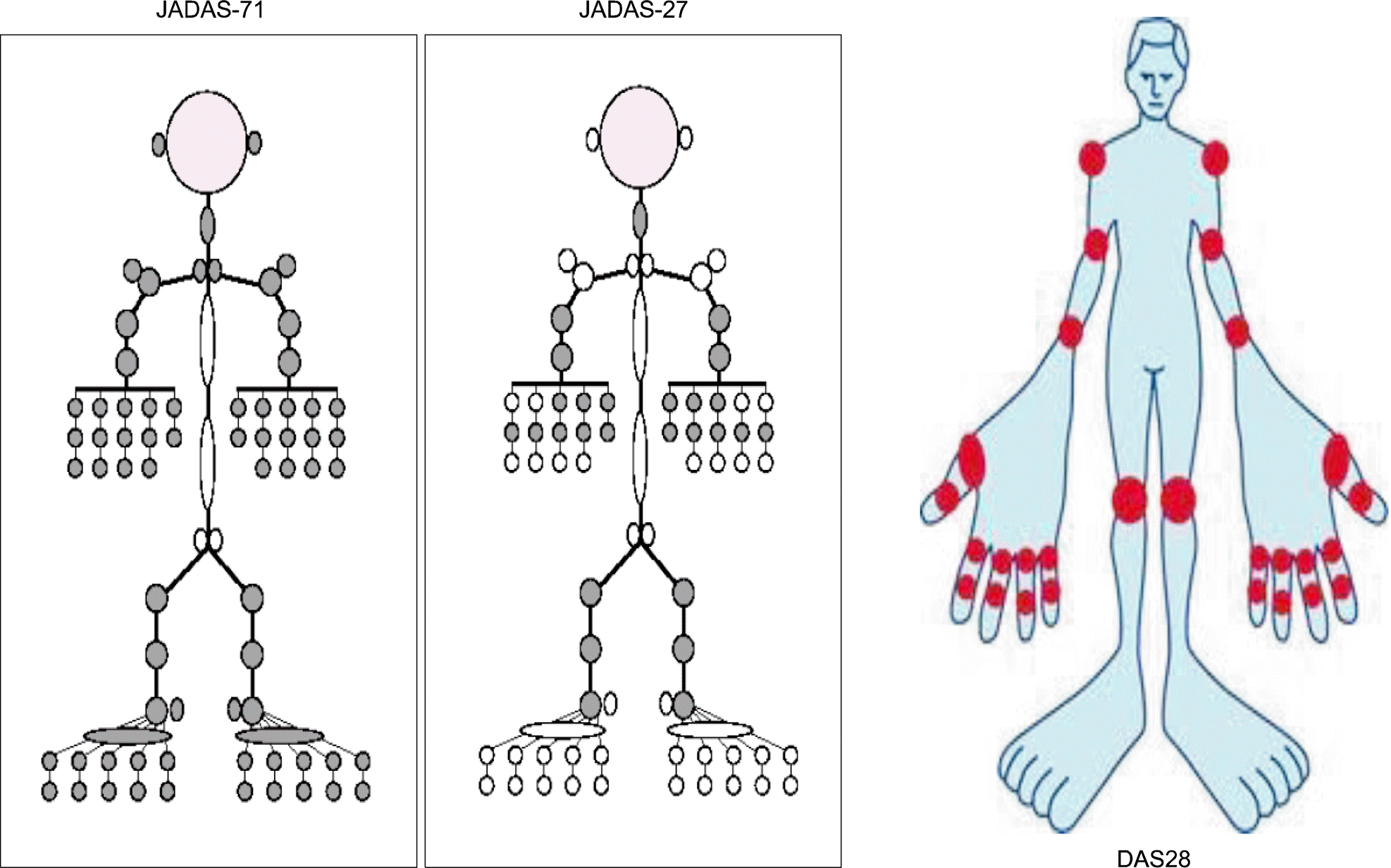 | Figure 1.The evaluable joint counts of juvenile arthritis disease activity score (JADAS) 71, JADAS 27 in pediatric arthritis, and DAS 28 in adult rheumatoid arthritis. |
Table 2.
Scoring form for the Juvenile Arthritis Functional Assessment Scale (JAFAS)
Table 3.
Composition and theoretical range in juvenile arthritis disease activity score (JADAS)
| JADAS-71 | JADAS-27 | JADAS-10 | |
|---|---|---|---|
| Physician global assessment | 0∼10 cm VAS | 0∼10 cm VAS | 0∼10 cm VAS |
| Parent/patient global assessment | 0∼10 cm VAS | 0∼10 cm VAS | 0∼10 cm VAS |
| Active joint count Simple | 0∼71 joints | Simple, 0∼27 joints | Simple, 0∼10 joints |
| Swollen joint count (range) | - | - | - |
| Tender joint count (range) | - | - | - |
| Acute phase reactant (range) | Normalized ESR* (0∼10) | Normalized ESR* (0∼10) | Normalized ESR* (0∼10) |
| Score range | 0∼101 | 0∼57 | 0∼40 |
Table 4.
Pain assessment under 3 years old




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download