Abstract
Objective
To evaluate the prevalence of sleep disturbance in Korean patients with ankylosing spondylitis (AS), and its association with disease activity and depression.
Methods
Forty patients with AS and eighty healthy controls were included in this study. Sleep quality was assessed using the Korean version of Pittsburgh sleep quality index (PSQI). Depression was assessed by the Korean version of Beck depression inventory second edition (BDI-2). Ankylosing spondylitis disease activity score-C-reactive protein (ASDAS-CRP) was used to evaluate disease activity. Patients were dichotomized into a good sleeper group (PSQI≤5) and a poor sleeper group (PSQI>5).
Results
The mean total PSQI score of patients with AS was 7.23±3.84. It was higher than that of the control subjects. AS patients had higher scores in all of the PSQI components, except for the use of sleep medication. Sixty percent of the AS patients were classified as poor sleepers. The mean BASDAI, ASDAS-CRP, and BDI-2 scores of the poor sleeper group were higher than that of the good sleeper group. Significantly, higher disease activity according to ASDAS-CRP was associated with poor sleep quality and depression. Multiple regression analysis revealed that the duration of morning stiffness and depression were independent risk factors that influenced poor sleep quality.
Go to : 
REFERENCES
1. Sieper J, Rudwaleit M, Khan MA, Braun J. Concepts and epidemiology of spondyloarthritis. Best Pract Res Clin Rheumatol. 2006; 20:401–17.

2. Dougados M, van der Linden S, Juhlin R, Huitfeldt B, Amor B, Calin A, et al. The European Spondylarthropathy Study Group preliminary criteria for the classification of spondylarthropathy. Arthritis Rheum. 1991; 34:1218–27.

3. Heiberg T, Lie E, van der Heijde D, Kvien TK. Sleep problems are of higher priority for improvement for patients with ankylosing spondylitis than for patients with other inflammatory arthropathies. Ann Rheum Dis. 2011; 70:872–3.

4. Hultgren S, Broman JE, Gudbjörnsson B, Hetta J, Lindqvist U. Sleep disturbances in outpatients with ankylosing spondylitisa questionnaire study with gender implications. Scand J Rheumatol. 2000; 29:365–9.
5. Hakkou J, Rostom S, Mengat M, Aissaoui N, Bahiri R, Hajjaj-Hassouni N. Sleep disturbance in Moroccan patients with ankylosing spondylitis: prevalence and relationships with disease-specific variables, psychological status and quality of life. Rheumatol Int. 2013; 33:285–90.

6. Kim TJ, Oh KT, Ju EK, Lee HS, Kim TH, Jun JB, et al. Health-related quality of life in Korean patients with ankylosing spondylitis. J Korean Rheum Assoc. 2002; 9(Suppl):S106–16.
7. van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984; 27:361–8.
8. Sohn SI, Kim do H, Lee MY, Cho YW. The reliability and validity of the Korean version of the Pittsburgh Sleep Quality Index. Sleep Breath. 2012; 16:803–12.

9. Garrett S, Jenkinson T, Kennedy LG, Whitelock H, Gaisford P, Calin A. A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol. 1994; 21:2286–91.
10. Lukas C, Landewé R, Sieper J, Dougados M, Davis J, Braun J, et al. Assessment of SpondyloArthritis international Society. Development of an ASAS-endorsed disease activity score (ASDAS) in patients with ankylosing spondylitis. Ann Rheum Dis. 2009; 68:18–24.

11. Machado P, Landewé R, Lie E, Kvien TK, Braun J, Baker D, et al. Assessment of SpondyloArthritis international Society. Ankylosing Spondylitis Disease Activity Score (ASDAS): defining cutoff values for disease activity states and improvement scores. Ann Rheum Dis. 2011; 70:47–53.

12. Lim SY, Lee EJ, Jeong SW, Kim HC, Jeong CH, Jeon TY, et al. The validation study of Beck Depression Scale 2 in Korean version. Anxiety Mood. 2011; 7:48–53.
13. Ohayon MM, Hong SC. Prevalence of insomnia and associated factors in South Korea. J Psychosom Res. 2002; 53:593–600.

14. Li Y, Zhang S, Zhu J, Du X, Huang F. Sleep disturbances are associated with increased pain, disease activity, depression, and anxiety in ankylosing spondylitis: a case-control study. Arthritis Res Ther. 2012; 14:R215.

15. Batmaz İ, Sarı yı ldız MA, Dilek B, Bez Y, Karakoç M, Çevik R. Sleep quality and associated factors in ankylosing spondylitis: relationship with disease parameters, psychological status and quality of life. Rheumatol Int. 2013; 33:1039–45.

16. Da Costa D, Zummer M, Fitzcharles MA. Determinants of sleep problems in patients with spondyloarthropathy. Musculoskeletal Care. 2009; 7:143–61.

17. Braun J, Bollow M, Neure L, Seipelt E, Seyrekbasan F, Herbst H, et al. Use of immunohistologic and in situ hy-bridization techniques in the examination of sacroiliac joint biopsy specimens from patients with ankylosing spondylitis. Arthritis Rheum. 1995; 38:499–505.

18. Lange U, Teichmann J, Stracke H. Correlation between plasma TNF-alpha, IGF-1, biochemical markers of bone metabolism, markers of inflammation/disease activity, and clinical manifestations in ankylosing spondylitis. Eur J Med Res. 2000; 5:507–11.
19. François RJ, Neure L, Sieper J, Braun J. Immunohistological examination of open sacroiliac biopsies of patients with ankylosing spondylitis: detection of tumour necrosis factor alpha in two patients with early disease and transforming growth factor beta in three more advanced cases. Ann Rheum Dis. 2006; 65:713–20.
21. Chennaoui M, Sauvet F, Drogou C, Van Beers P, Langrume C, Guillard M, et al. Effect of one night of sleep loss on changes in tumor necrosis factor alpha (TNF-α) levels in healthy men. Cytokine. 2011; 56:318–24.

Go to : 
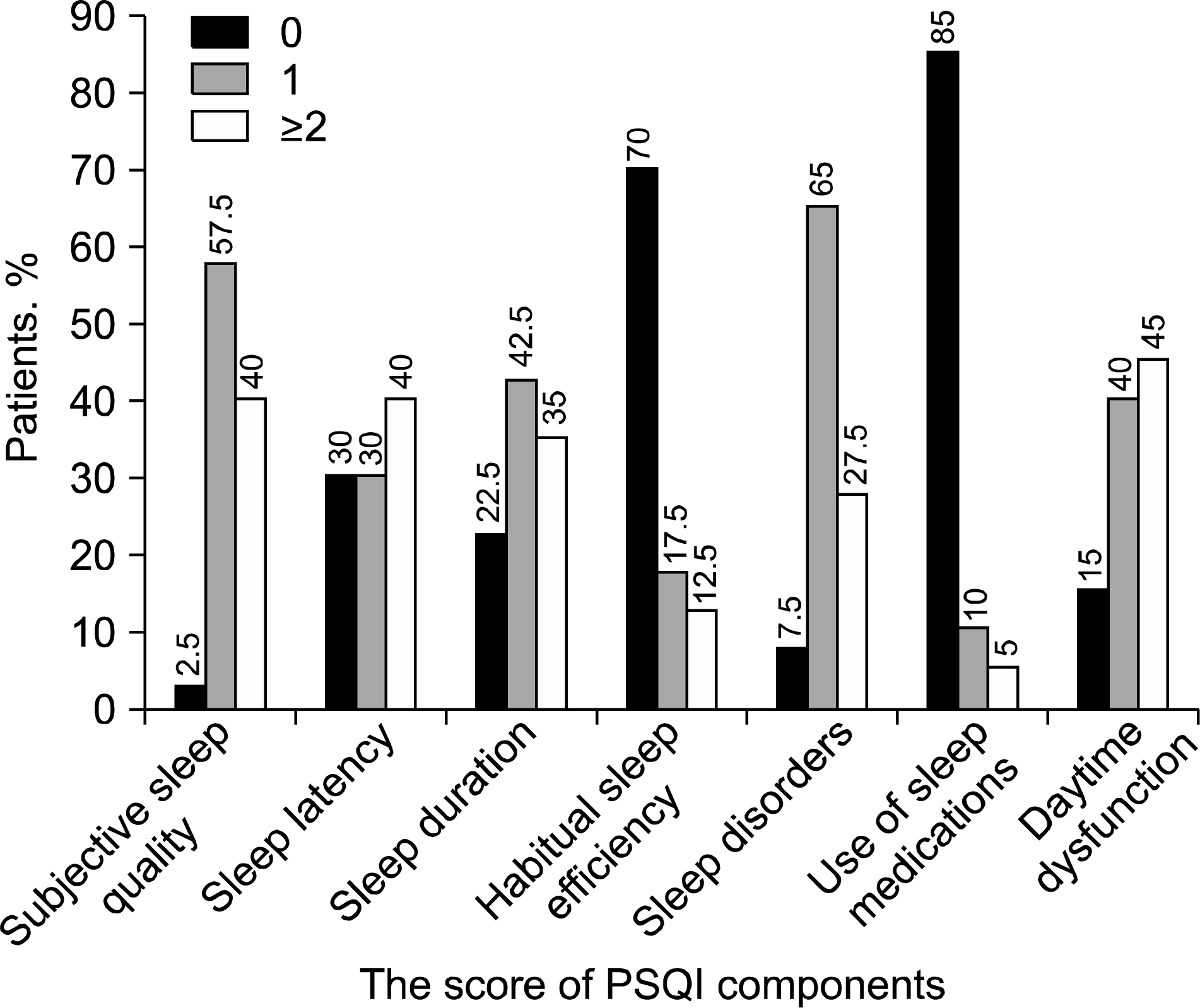 | Figure 1.Score distribution of each PSQI component in patients with ankylosing spondylitis. |
Table 1.
Comparison between patient group and control group
| Patient group (n=40) | Control group (n=80) | p | |
|---|---|---|---|
| Age, years | 42.18±11.95 | 43.13±12.97 | 0.699* |
| Male, n (%) | 34 (85%) | 33 (41.3%) | <0.001† |
| Disease duration, months | 71.97±73.80 | NA | |
| HLA-B27 positivity, n (%) | 33 (82.5%) | NA | |
| TNF-α blocker use, n (%) | 25 (62.5%) | NA | |
| CRP, mg/L | 3.20±5.82 | NA | |
| ASDAS-CRP | 1.93±0.85 | NA | |
| BASDAI | 3.67±2.07 | NA | |
| BDI-2 | 14.18±11.41 | 6.31±6.16 | <0.001* |
| Total PSQI | 7.23±3.84 | 3.80±2.02 | <0.001* |
| Subjective sleep quality | 1.40±0.59 | 0.84±0.60 | <0.001* |
| Sleep latency | 1.35±1.23 | 0.71±0.73 | 0.004* |
| Sleep duration | 1.20±0.88 | 0.70±0.80 | 0.002* |
| Habitual sleep efficiency | 0.45±0.78 | 0.11±0.42 | 0.014* |
| Sleep disturbance | 1.20±0.56 | 0.73±0.52 | <0.001* |
| Use of sleep medications | 0.25±0.70 | 0.04±0.24 | 0.072* |
| Daytime dysfunction | 1.40±0.87 | 0.68±0.68 | <0.001* |
| Good sleepers (PSQI≤5) | 16 (40%) | 67 (83.8%) | <0.001† |
| Poor sleepers (PSQI>5) | 24 (60%) | 13 (16.3%) |
Table 2.
Comparison between good sleepers and poor sleepers in the patient group
| Variables | Good sleepers (PSQI≤5) n=16 | Poor sleepers (PSQI>5) n=24 | p |
|---|---|---|---|
| Age, years | 43.06±11.21 | 41.58±12.61 | 0.795* |
| Disease duration, months | 59.69±52.81 | 80.17±85.10 | 0.733* |
| Male, n (%) | 15 (93.8%) | 19 (79.2%) | 0.373† |
| HLA-B27 positivity, n (%) | 14 (87.5%) | 19 (79.2%) | 0.818† |
| TNF-α blocker use, n (%) | 11 (68.8%) | 14 (58.3%) | 0.740† |
| BASDAI | 2.10±1.59 | 4.72±1.66 | <0.001* |
| Fatigue | 2.50±1.93 | 5.50±1.91 | <0.001* |
| Spinal pain | 2.69±2.12 | 4.88±2.30 | 0.005* |
| Peripheral joint pain/swelling | 2.00±1.75 | 4.71±2.61 | 0.001* |
| Tenderness | 1.38±1.82 | 3.33±2.54 | 0.008* |
| Severity of morning stiffness | 2.38±1.99 | 4.79±2.46 | 0.003* |
| Duration of morning stiffness | 1.56±1.26 | 5.42±3.34 | <0.001* |
| ASDAS-CRP | 1.36±0.78 | 2.31±0.68 | <0.001* |
| CRP, mg/L | 4.00±8.44 | 2.68±3.22 | 0.795* |
| Spinal pain | 2.69±2.12 | 4.88±2.30 | 0.005* |
| Duration of morning stiffness | 1.56±1.26 | 5.42±3.34 | <0.001* |
| Patient global status | 2.38±1.40 | 5.04±2.09 | <0.001* |
| Peripheral joint pain/swelling | 2.00±1.75 | 4.71±2.61 | 0.001* |
| BDI-2 | 7.00±6.38 | 18.96±11.59 | <0.001* |
Table 3.
Sleep quality of patients with ankylosing spondylitis according to disease activity (ASDAS-CRP)
| Inactive (ASDAS<1.3) N=11 | Moderate (1.3≤ ASDAS<2.1) N=9 | High (ASDAS≥2.1) N=20 | p | |
|---|---|---|---|---|
| Age, years | 41.27±10.99 | 48.22±11.9983.67±60.81 | 39.95±12.08 | 0.282* |
| Duration, months | 69.64±53.01 | 83.67±60.81 | 68.00±89.77 | 0.333* |
| Male, n (%) | 11 (100%) | 6 (66.7%)7 (77.8%) | 17 (85.0%) | 0.118† |
| HLA-B27 positivity, n (%) | 10 (90.9%) | 7 (77.8%)6 (66.7%) | 16 (80.0%) | 0.728† |
| TNF-α blocker use, n (%) | 9 (81.8%) | 6 (66.7%)2.13±3.01 | 10 (50.0%) | 0.205† |
| CRP, mg/L | 0.48±0.80 | 2.13±3.013.17±1.60 | 5.19±7.51 | 0.007* |
| BASDAI | 1.75±0.88 | 3.17±1.605.22±3.07 | 4.95±1.82 | <0.001* |
| Total PSQI | 4.91±1.86 | 5.22±3.071.33±0.70 | 9.40±3.81 | 0.001* |
| Subjective sleep quality | 1.09±0.30 | 1.33±0.700.67±1.00 | 1.60±0.59 | 0.057* |
| Sleep latency | 0.73±0.64 | 0.67±1.000.89±0.78 | 2.00±1.25 | 0.003* |
| Sleep duration | 0.82±0.75 | 0.89±0.780.11±0.33 | 1.55±0.88 | 0.051* |
| Habitual sleep efficiency | 0.09±0.30 | 0.11±0.33 | 0.80±0.95 | 0.017* |
| Sleep disturbances | 0.91±0.30 | 0.89±0.33 | 1.50±0.60 | 0.002* |
| Use of sleep medications | 0.45±0.93 | 0 | 0.25±0.71 | 0.247* |
| Daytime dysfunction | 0.82±0.75 | 1.33±1.00 | 1.75±0.71 | 0.019* |
| BDI-2 | 5.64±6.07 | 14.56±7.07 j | 18.70±12.76 | 0.004* |
Table 4.
Correlation coefficients between components of PSQI and demographics and disease-related variables
| Variables | Total PSQI | Subjective sleep quality | Sleep latency | Sleep duration | Habitual sleep efficiency | Sleep disturbance | Use of sleep medication | Daytime dysfunction |
|---|---|---|---|---|---|---|---|---|
| Age | −0.115 | −0.014 | −0.274 | 0.023 | −0.058 | −0.066 | 0.004 | −0.044 |
| Disease duration | 0.236 | 0.298 | 0.088 | 0.316* | 0.247 | 0.146 | 0.035 | 0.137 |
| BASDAI | 0.691† | 0.638† | 0.490† | 0.553† | 0.354* | 0.683† | 0.022 | 0.642† |
| Fatigue | 0.690† | 0.580† | 0.415† | 0.574† | 0.334* | 0.596† | 0.151 | 0.711† |
| Spinal pain | 0.439† | 0.547† | 0.327* | 0.354* | 0.120 | 0.555† | −0.147 | 0.443† |
| Peripheral joint pain/swellin | g 0.604† | 0.607† | 0.404† | 0.559† | 0.331* | 0.601† | 0.010 | 0.456† |
| Tenderness | 0.366* | 0.339* | 0.341* | 0.196 | 0.108 | 0.492† | −0.037 | 0.361* |
| Severity of morning stiffnes | s 0.657† | 0.539† | 0.441† | 0.549† | 0.437† | 0.575† | 0.067 | 0.584† |
| Duration of morning stiffnes | ss 0.674† | 0.434† | 0.485† | 0.519† | 0.529† | 0.496† | 0.157 | 0.625† |
| ASDAS-CRP | 0.557† | 0.446† | 0.471† | 0.400* | 0.486† | 0.504† | −0.122 | 0.491† |
| CRP | −0.151 | −0.161 | −0.109 | −0.075 | 0.093 | −0.319* | −0.124 | −0.113 |
| Spinal pain | 0.439† | 0.547† | 0.327* | 0.354* | 0.120 | 0.555† | −0.147 | 0.443† |
| Duration of morning stiffnes | ss 0.674* | 0.434† | 0.485† | 0.519† | 0.529† | 0.496† | 0.157 | 0.625† |
| Patient global status | 0.620† | 0.507† | 0.482† | 0.401* | 0.384* | 0.628† | 0.004 | 0.605† |
| Peripheral joint pain/swelling | 0.604† | 0.607† | 0.404† | 0.559† | 0.331* | 0.601† | 0.010 | 0.456† |
| BDI-2 | 0.742† | 0.644† | 0.478† | 0.531† | 0.508† | 0.568† | 0.277 | 0.583† |
Table 5.
Stepwise multiple regression analysis between components of PSQI and demographics and disease-related variables




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download