Abstract
Vasculitis that involves the gastrointestinal (GI) tract often occurs as part of a systemic inflammatory process. It is a well-recognized manifestation of the small and medium sized vessel vasculitides. Vasculitis of the GI tract may occur in isolation; although it can progress to a systemic illness. It usually involves the arterioles, venules, and capil-laries; however, it is very rare for only the venules to be affected. Enterocolic lymphocytic phlebitis is a localized vasculitis, typically affecting the small and medium-sized intramural and mesenteric veins of the intestines. We report a case of enterocolic lymphocytic phlebitis of the colon. A 38-year-old woman was presented with hematochezia and severe abdominal pain on the day of admission. She had no history of intestinal disease or systemic disease. Computed tomography showed an extremely thickened wall of the colon, along with several air bubbles in the colon with diffuse subcutaneous emphysema in the abdominal wall. An emergency exploration laparotomy and extended right hemicolectomy was performed. The patient recovered completely after surgery and remains well without further therapy.
Go to : 
REFERENCES
1. Ronald AS, Russell GP. Townsend: Sabiston Textbook of Surgery. 19th ed.p. 1141. Philadelphia, Saunders: An im-print of Elsevier;2012.
2. Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013; 65:1–11.

3. Pagnoux C, Mahr A, Cohen P, Guillevin L. Presentation and outcome of gastrointestinal involvement in systemic necrotizing vasculitides: analysis of 62 patients with polyarteritis nodosa, microscopic polyangiitis, Wegener granulomatosis, Churg-Strauss syndrome, or rheumatoid arthritis-associated vasculitis. Medicine (Baltimore). 2005; 84:115–28.
4. Gonzalez-Gay MA, Vazquez-Rodriguez TR, Miranda-Filloy JA, Pazos-Ferro A, Garcia-Rodeja E. Localized vasculitis of the gastrointestinal tract: a case report and literature review. Clin Exp Rheumatol. 2008; 26(3 Suppl 49):S101–4.
5. Salvarani C, Calamia KT, Crowson CS, Miller DV, Broadwell AW, Hunder GG, et al. Localized vasculitis of the gastrointestinal tract: a case series. Rheumatology (Oxford). 2010; 49:1326–35.

6. Atisha-Fregoso Y, Hinojosa-Azaola A, Alcocer-Varela J. Localized, single-organ vasculitis: clinical presentation and management. Clin Rheumatol. 2013; 32:1–6.

7. Ahn E, Luk A, Chetty R, Butany J. Vasculitides of the gastrointestinal tract. Semin Diagn Pathol. 2009; 26:77–88.

8. Garcia-Porrua C, Gutierrez-Duque O, Soto S, Garcia-Rodeja E, Gonzalez-Gay MA. Localized vasculitis of the gastrointestinal tract. Semin Arthritis Rheum. 2006; 35:403–6.

9. Herná ndez-Rodríguez J, Hoffman GS. Updating singleorgan vasculitis. Curr Opin Rheumatol. 2012; 24:38–45.
10. Burke AP, Sobin LH, Virmani R. Localized vasculitis of the gastrointestinal tract. Am J Surg Pathol. 1995; 19:338–49.

11. Ngo N, Chang F. Enterocolic lymphocytic phlebitis: clinicopathologic features and review of the literature. Arch Pathol Lab Med. 2007; 131:1130–4.

12. Jain R, Chetty R. Enterocolic lymphocytic phlebitis and lymphocytic colitis: drug-related coexistent pathology. Int J Colorectal Dis. 2009; 24:473–4.

13. Saraga E, Bouzourenne H. Enterocolic (lymphocytic) phlebitis: a rare cause of intestinal ischemic necrosis: a series of six patients and review of the literature. Am J Surg Pathol. 2000; 24:824–9.
Go to : 
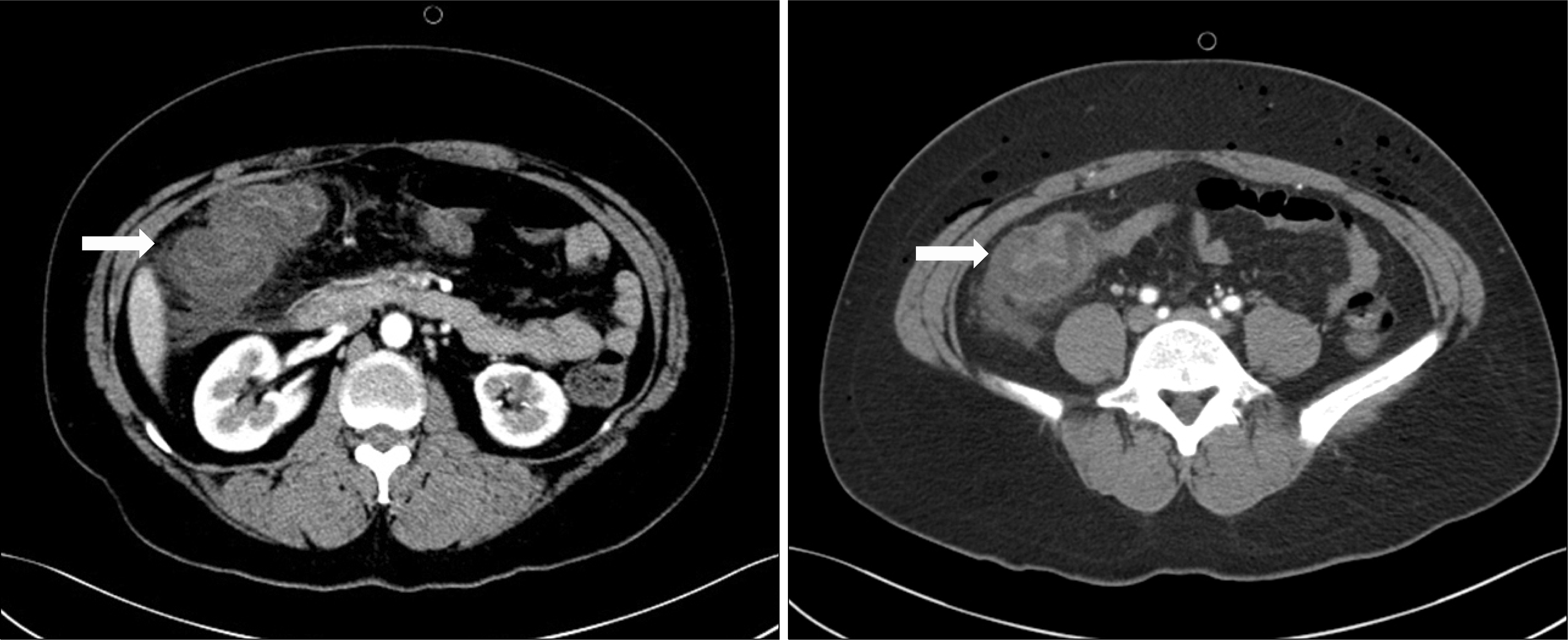 | Figure 1.Abdominal CT shows an extremely thickened wall of the ascending colon (arrow) with diffuse scattered subcutaneous emphysema in the abdominal wall. |
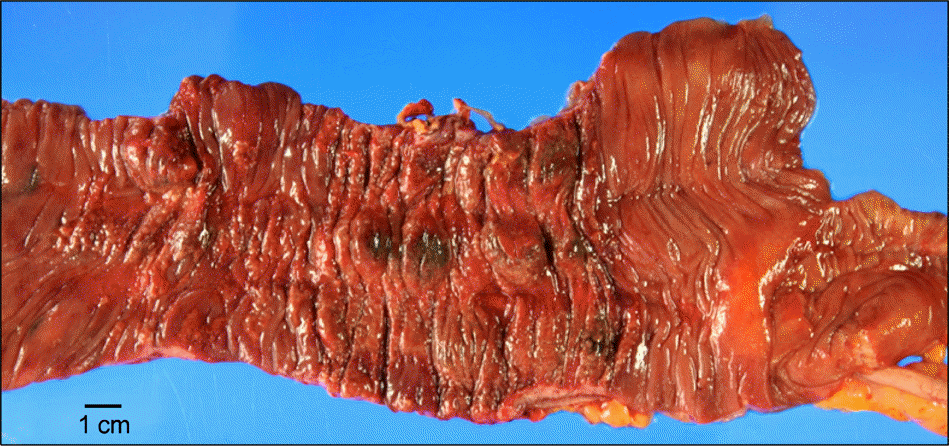 | Figure 2.Gross features of the specimen. An ill defined lesion with irregular surface is localized in ascending colon. The mucosa of the lesion is necrotic, stiff and reddish. |
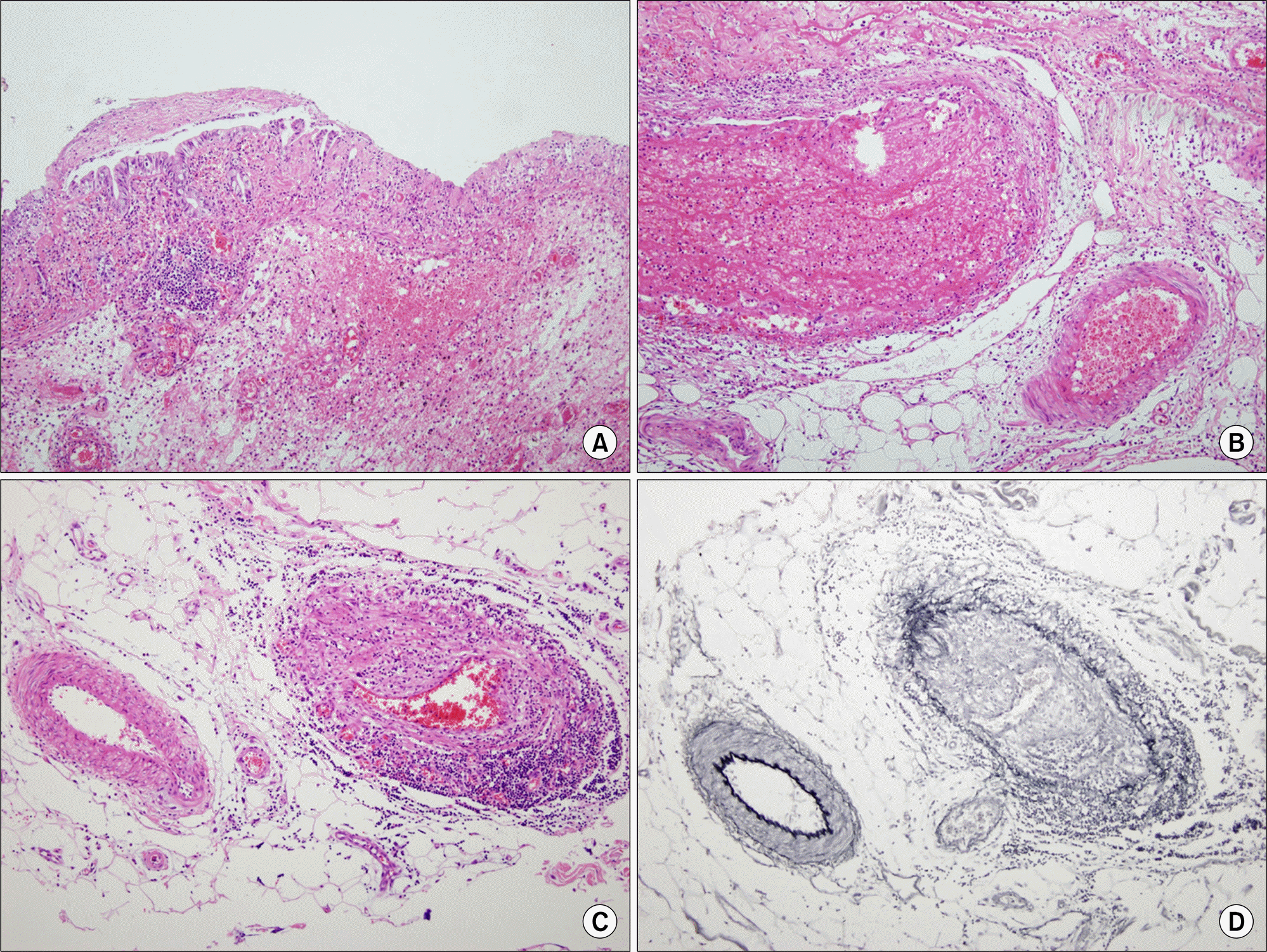 | Figure 3.Microscopic findings of enterocolic phlebitis. The lesional mucosa and submucosa are shown in (A). The mucosa reveals ischemic necrosis and the submucosa is hemorrhagic and edematous. The submucosal veins of the lesion (B) are filled with thrombi, and show necrotizing vasculitis with fibrinoid degeneration. The vessels at the periphery (C and D) show lymphocytic phlebitis, relatively. Special stain for elastin (D) demonstrated that arteries are spared. (A-C, Hematoxylin-eosin stain ×200; D, Elastic stain ×200). |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download