Abstract
Objective
Methotrexate is the first-line drug in treatment of rheumatoid arthritis (RA) exhibiting higher efficacy and better tolerability than most other DMARDs. To have a better understanding of the antiarthritic mechanism of methotrexate, we investigated the effect of methotrexate on suppressing the autoimmune inflammatory and destructive arthritis in collagen-induced arthritis (CIA) mice.
Methods
The effects of methotrexate on joint inflammation were assessed by clinical scoring and histologic analysis. Levels of cytokines and autoreactive antibodies were analyzed by immunohistochemistry and ELISA. The population of TH17 and Foxp3+ regulatory T (Treg) cells and phosphorylation of their critical transcription activators, STAT3 and STAT5, were examined by fluorescence micro-scopy and flow cytometry, respectively.
Results
Treatment with methotrexate significantly alle-viated joint inflammation and cartilage destruction in CIA. Serum levels of total immunoglobulins G, G1, G2a specific to type II collagen were also reduced considerably in methotrexate-treated mice. The drug inhibited the expression of proinflammatory cytokines such as IL-1β, TNF-α, IL-6 and IL-17 in arthritic joints ex vivo as well as by splenocytes in vitro. Moreover, methotrexate treatment resulted in reciprocal modulation of TH17 cells and Foxp3+ regulatory T (Treg) cells in spleen tissues, in which TH17 cells were decreased and Treg cells in number were increased. Subsequent analysis of CD4+ T cells showed that phosphorylation of STAT3 was decreased whereas phosphorylation of STAT5 was increased in methotrexate-treated mice.
Go to : 
References
1. Firestein GS, Kelley WN. Kelley's textbook of rheumatology. Cannella AC, O'dell JR, editors. Methotrexate, leflunomide, sulfasalazine, hydroxychloroquine, and combination therapies. 8th ed.p. 883. Philadelphia, PA: Saunders Elsevier;2009.
2. Link AA, Kino T, Worth JA, McGuire JL, Crane ML, Chrousos GP, et al. Ligand-activation of the adenosine A2a receptors inhibits IL-12 production by human monocytes. J Immunol. 2000; 164:436–42.

3. Haskó G, Szabó C, Németh ZH, Kvetan V, Pastores SM, Vizi ES. Adenosine receptor agonists differentially regulate IL-10, TNF-alpha, and nitric oxide production in RAW 264.7 macrophages and in endotoxemic mice. J Immunol. 1996; 157:4634–40.
4. Mello SB, Barros DM, Silva AS, Laurindo IM, Novaes GS. Methotrexate as a preferential cyclooxygenase 2 inhibitor in whole blood of patients with rheumatoid arthritis. Rheumatology (Oxford). 2000; 39:533–6.

5. Chen Z, Laurence A, Kanno Y, Pacher-Zavisin M, Zhu BM, Tato C, et al. Selective regulatory function of Socs3 in the formation of IL-17-secreting T cells. Proc Natl Acad Sci U S A. 2006; 103:8137–42.

6. Durant L, Watford WT, Ramos HL, Laurence A, Vahedi G, Wei L, et al. Diverse targets of the transcription factor STAT3 contribute to T cell pathogenicity and homeostasis. Immunity. 2010; 32:605–15.

7. Yang XP, Ghoreschi K, Steward-Tharp SM, Rodriguez-Canales J, Zhu J, Grainger JR, et al. Opposing regulation of the locus encoding IL-17 through direct, reciprocal actions of STAT3 and STAT5. Nat Immunol. 2011; 12:247–54.

8. Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, et al. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008; 453:236–40.
9. Elias KM, Laurence A, Davidson TS, Stephens G, Kanno Y, Shevach EM, et al. Retinoic acid inhibits Th17 polarization and enhances FoxP3 expression through a Stat-3/Stat-5 independent signaling pathway. Blood. 2008; 111:1013–20.

10. Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007; 317:256–60.
11. Li Y, Jiang L, Zhang S, Yin L, Ma L, He D, et al. Methotrexate attenuates the Th17/IL-17 levels in peripheral blood mononuclear cells from healthy individuals and RA patients. Rheumatol Int. 2012; 32:2415–22.

12. Fiehn C, Wunder A, Krienke S, Max R, Ho AD, Moehler T. Lack of evidence for inhibition of angiogenesis as a central mechanism of the antiarthritic effect of methotrexate. Rheumatol Int. 2005; 25:108–13.

13. Barnett ML, Kremer JM, St Clair EW, Clegg DO, Furst D, Weisman M, et al. Treatment of rheumatoid arthritis with oral type II collagen. Results of a multicenter, double-blind, placebo-controlled trial. Arthritis Rheum. 1998; 41:290–7.

14. Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986; 136:2348–57.
15. Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005; 6:1123–32.

16. Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005; 6:1133–41.

17. Dong C. Differentiation and function of proinflammatory Th17 cells. Microbes Infect. 2009; 11:584–8.

18. Tesmer LA, Lundy SK, Sarkar S, Fox DA. Th17 cells in human disease. Immunol Rev. 2008; 223:87–113.

20. Iwakura Y, Nakae S, Saijo S, Ishigame H. The roles of IL-17A in inflammatory immune responses and host de-fense against pathogens. Immunol Rev. 2008; 226:57–79.

21. Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004; 22:531–62.

22. Sakaguchi S, Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T. Regulatory T cells: how do they suppress immune responses? Int Immunol. 2009; 21:1105–11.

24. Oh JS, Kim YG, Lee SG, So MW, Choi SW, Lee CK, et al. The effect of various disease-modifying antirheumatic drugs on the suppressive function of CD4+ CD25+ regulatory T cells. Rheumatol Int. 2013; 33:381–8.
Go to : 
 | Figure 1.Suppression of arthritis development in MTX-treated CIA mice. Arthritis was induced by immunization with CII in Freund's complete adjuvant on day 0. On day 7, mice also received PBS or MTX (1 mg/kg or 7.5 mg/kg) three times per week for 7 weeks. (A) Severity of arthritis was determined as described in Materials and Methods. (B) Representative histological analysis of knee joints and paws was assessed by H & E, toluidine blue, and safranin o staining. Original magnification: ×40 and ×200 for H&E staining and ×200 for toluidine blue and safranin o staining. Values of histological scores of inflammation and cartilage damage were shown in the right panel. (C) Anti-collagen antibody levels in CIA mice. The levels of IgG anti-collagen antibodies were measured by ELISA. Values are expressed as the optical density (O.D.) ∗p<0.01, † p<0.001 compared to the vehicle control mice. |
 | Figure 2.Immunohistologies of joints tissues from MTX treated CIA mice. Tissue sections from mice joints of each group were stained with anti-TNF-α, anti-IL-1β, anti-IL-6, anti-IL-17 antibodies or isotype antibodies, respectively. Cells stained with each antibody are shown in brown. Original magnification: ×400. |
 | Figure 3.Reciprocal regulation of TH17 and Foxp3+ regulatory T cells in MTX-treated CIA mice. Spleen from vehicle and MTX-treated CIA mice were stained by anti-CD4 (green), anti-CD25 (blue), and anti-IL-17 (red, upper), anti-Foxp3 (red, lower) antibodies. Populations of CD4+ CD25+ Foxp3+ T cells and CD4+ IL-17+ T cells were analyzed using laser confocal mi-croscopy. Original magnification: ×400. The graphs represent the number of positive cells. ∗p<0.05 compared to the vehicle control mice. |
 | Figure 4.STATs-dependent regulation of TH17 and Foxp3+ regulatory T cells in MTX-treated CIA mice. Spleens from mice in each group were stained with antibodies against anti-CD4 (green, upper; white, lower), anti-Foxp3 (red), phos-pho-STAT3 (red) or phospho-STAT5 (blue). Original magnification: ×400. The graphs represent the number of positive cells. Data are expressed as the mean± SD. ∗p<0.05 compared to the vehicle control mice. |
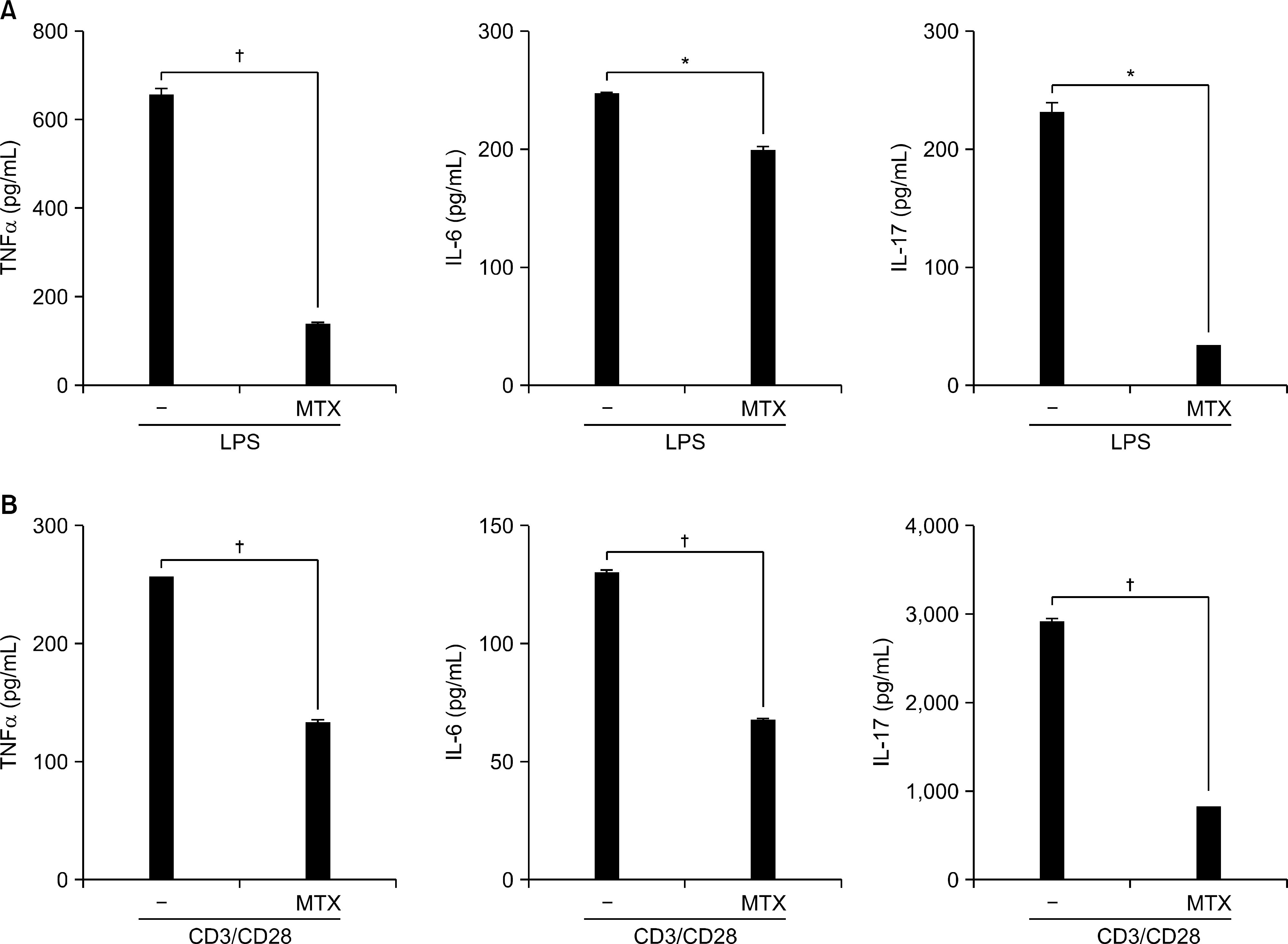 | Figure 5.Suppressive effect of MTX on production of inflammatory cytokines. Splenocytes of DBA/1J mice were cultured with LPS (100 ng/mL) (A) or anti-CD3 (1 μ g/mL)/CD28 (2 μ g/mL) (B) in the presence or absence of MTX (0.5 μ g/mL) for 24 hours in vitro. TNF-α, IL-6 and IL-17 in the cell supernatant was determined by ELISA. Data are expressed as the mean± SD. ∗p<0.01, † p <0.001 compared to the LPS or CD3/CD28-treated cells. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download