Abstract
Objective
Kawasaki disease is a systemic vascular disease which is caused by an immunologic response. The purpose of this study is to see how a high IgE level affects Kawasaki disease, in two groups of high IgE level and low IgE level.
Methods
A retrospective study was done from 2008 to 2010, among patients, who were admitted in Severance Children's Hospital for Kawasaki disease with IgE levels checked. Age groups with an IgE level above reference ranges and those with normal ranges were done. Also, clinical characteristics were analyzed. Statistical method was done by SPSS 18.
Results
A total of 198 Kawasaki patients were analyzed from 2008 to 2010. Among them 123 (62%) patients showed elevated IgE levels. Patients with high IgE had a significantly higher lymphadenopathy prevalence (p=0.006), however they had no connection with quantitative values. Patients with BCG site redness appeared to have lower IgE levels than patients without redness. Coronary complication had no relation with IgE levels. There was no correlation between laboratory results and IgE levels.
Go to : 
References
1. Taubert KA, Rowley AH, Shulman ST. Nationwide survey of Kawasaki disease and acute rheumatic fever. J Pediatr. 1991; 119:279–82.

2. Kawasaki T. Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children. Arerugi. 1967; 16:178–222.
3. JCS Joint Working Group. Guidelines for diagnosis and management of cardiovascular sequelae in Kawasaki disease (JCS 2008)–digest version. Circ J. 2010; 74:1989–2020.
4. Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, Burns JC, et al. Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease Council on Cardiovascular Disease in the Young American Heart Association American Academy of Pediatrics. Diagnosis, treatment, and longterm management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. 2004; 110:2747–71.

7. Yanagawa H, Nakamura Y, Yashiro M, Oki I, Hirata S, Zhang T, et al. Incidence survey of Kawasaki disease in 1997 and 1998 in Japan. Pediatrics. 2001; 107:E33.

8. Webster RJ, Carter KW, Warrington NM, Loh AM, Zaloumis S, Kuijpers TW, et al. Hospitalisation with infection, asthma and allergy in Kawasaki disease patients and their families: genealogical analysis using linked population data. PLoS One. 2011; 6:e28004.

9. Kusakawa S, Heiner DC. Elevated levels of immunoglobulin E in the acute febrile mucocutaneous lymph node syndrome. Pediatr Res. 1976; 10:108–11.

10. Matsuoka S, Tatara K, Nakagawa R, Mori K, Kuroda Y. Tendency toward atopy in Kawasaki disease. Eur J Pediatr. 1997; 156:30–2.

11. Furukawa S, Matsubara T, Motohashi T, Sasai K, Nakachi S, Umezawa Y, et al. Increased expression of Fc epsilonR2/CD23 on peripheral blood B lymphocytes and serum IgE levelsin Kawasaki disease. Int Arch Allergy Appl Immunol. 1991; 95:7–12.
12. Liew WK, Lim CW, Tan TH, Wong KY, Tai BC, Quek SC, et al. The effect of Kawasaki disease on childhood allergies - a sibling control study. Pediatr Allergy Immunol. 2011; 22:488–93.

13. Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, Burns JC, et al. Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Diagnosis, treatment, and longterm management of Kawasaki disease:a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Pediatrics. 2004; 114:1708–33.
14. Soldin SJ, Lenherr S, Kumar A. Pediatric reference ranges for IgE. Clin Chem. 1995; 41:S92.
15. McCloskey N, Hunt J, Beavil RL, Jutton MR, Grundy GJ, Girardi E, et al. Soluble CD23 monomers inhibit and oligomers stimulate IGE synthesis in human B cells. J Biol Chem. 2007; 282:24083–91.

16. Cooper AM, Hobson PS, Jutton MR, Kao MW, Drung B, Schmidt B, et al. Soluble CD23 controls IgE synthesis and homeostasis in human B cells. J Immunol. 2012; 188:3199–207.

17. Bonnefoy JY, Gauchat JF, Life P, Graber P, Aubry JP, Lecoanet-Henchoz S. Regulation of IgE synthesis by CD23/CD21 interaction. Int Arch Allergy Immunol. 1995; 107:40–2.

18. Uehara R, Igarashi H, Yashiro M, Nakamura Y, Yanagawa H. Kawasaki disease patients with redness or crust formation at the Bacille Calmette-Gué rin inoculation site. Pediatr Infect Dis J. 2010; 29:430–3.
19. Wang P, Zhang G, Qin X, Qiu Z, Li N, Chen Z, et al. Inhibition of allergen-induced airway remodeling by neonatal bacillus Calmette-Guerin vaccination is associated with interferon-gamma-producing T cells but not regulatory T cells in mice. Ann Allergy Asthma Immunol. 2011; 107:163–70.

20. Shen H, Huang H, Wang J, Ye S, Li W, Wang K, et al. Neonatal vaccination with Bacillus Calmette-Gué rin elic-its longterm protection in mouse-allergic responses. Allergy. 2008; 63:555–63.
21. Cavallo GP, Elia M, Giordano D, Baldi C, Cammarota R. Decrease of specific and total IgE levels in allergic patients after BCG vaccination: preliminary report. Arch Otolaryngol Head Neck Surg. 2002; 128:1058–60.
Go to : 
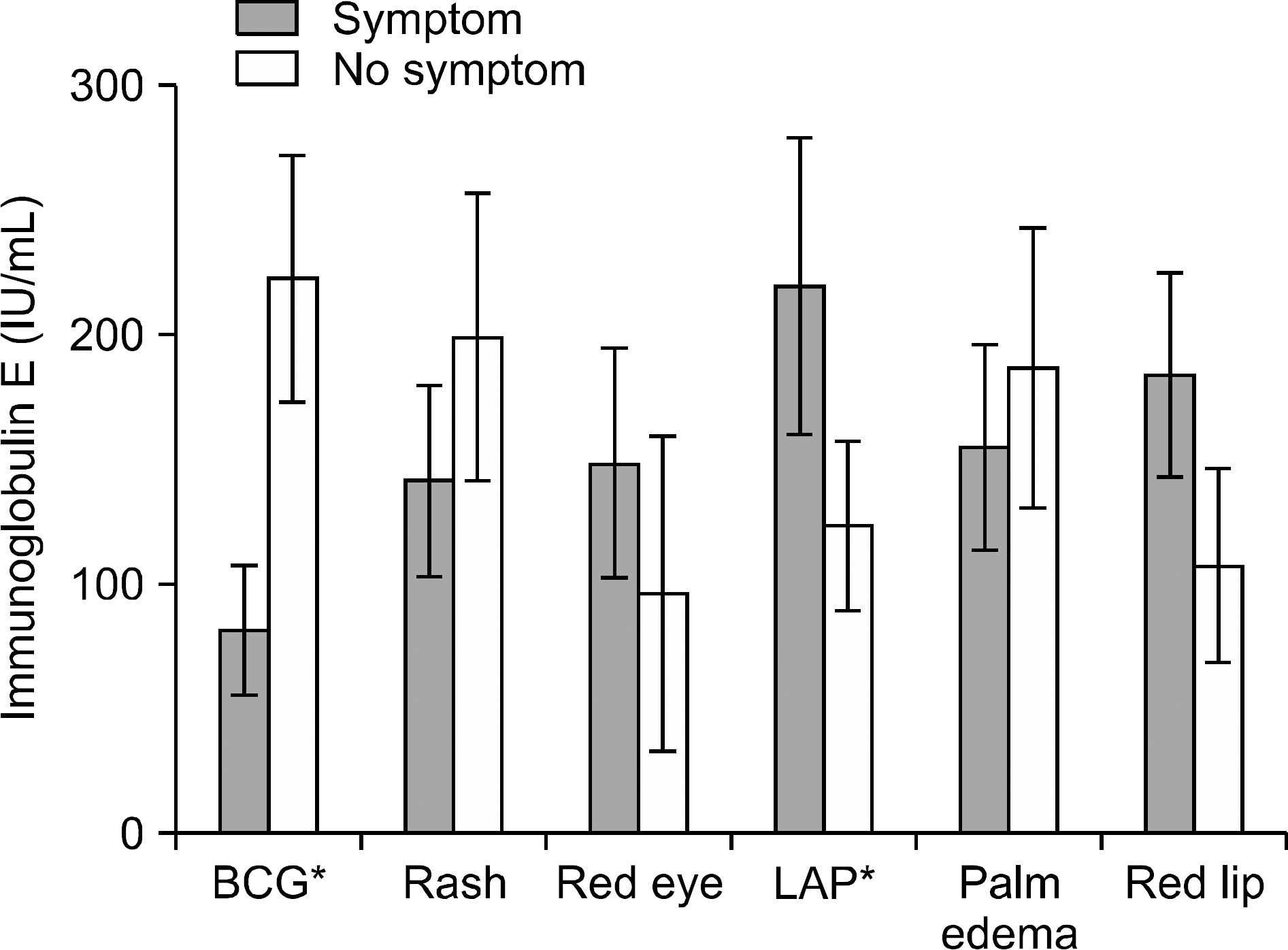 | Figure 1.Serum IgE by clinical manifestations. Patients with high IgE had significantly high lymphadenopathy prevalence and patients without BCG site injection appeared to have lower IgE levels than patients with injection. (∗p-value<0.05). Other symptoms had no relation with IgE levels. |
Table 1.
Reference values of serum IgE by age
| Age | Male (IU/mL) | Female (IU/mL) |
|---|---|---|
| 0∼12 months | <12 | <8 |
| 1∼3 years | <90 | <28 |
| 4∼10 years | <163 | <137 |
| 11∼18 years | <179 | <398 |
Table 2.
Patients’ mean serum IgE level by age
| Age | <1 years | 1∼3 years | >3 years |
|---|---|---|---|
| IgE (IU/mL) | 51.2±85.9 | 152.1±179.3 | 261.6±306.5 |
| Patients numbers with high IgE | 42/52 (80%) | 47/68 (69%) | 34/78 (44%) |
Table 3.
Laboratory characteristics of patients with IgE values
Table 4.
Analysis of clinical symptoms and IgE values in each age group




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download