Abstract
Objective
The idiopathic inflammatory myopathies (IIMs) are chronic systemic connective tissue diseases. The muscle biopsy is a definitive diagnostic tool but blind biopsy some-times produces to negative results. Magnetic resonance imaging (MRI) as a tool for early diagnosis, guidance for biopsy, assessing extent of lesions and monitoring therapy in IIMs has been reported. The aim of this study is to assess the association of thigh inflammation through MRI and biopsy specimens with clinical findings.
Methods
Sixty patients diagnosed with dermatomyositis (DM) or polymyositis (PM) from 2004 to 2011 in one center of rheumatology were enrolled. We reviewed clinical, laboratory, histopathologic and MRI of thigh data at initial diagnosis. The inflammation grades by MRI and histo-pathology of muscles were evaluated through 4-point scoring systems.
Results
The laboratory findings for aldolase and CK dif-fered significantly between DM patients (68.3%) and PM patients (31.7%). Fasciitis was detected by MRI in 43.3% of patients, of whom 88.5% had DM (p<0.05). The fasciitis was also associated with myalgia (p<0.05). Almost all MRI findings were symmetric except for two patients. The mean of total signal intensity was higher in patients with decreased muscle power. The signal intensity of affected muscle was slightly associated with muscle enzymes and histopathologic grading.
Conclusion
Fasciitis was observed more in DM patients. MRI findings were associated with muscle enzymes and histopathologic grading. Signal intensity on MRI may be useful for measurement of disease activity in acute IIMs. The noninvasive nature and high sensitivity of muscle inflammation suggest that MRI images should be considered prior to muscle biopsy and treatment of IIMs.
References
1. Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med. 1975; 292:344–7.
2. Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts). N Engl J Med. 1975; 292:403–7.
3. Dalakas MC. Polymyositis, dermatomyositis and in-clusion-body myositis. N Engl J Med. 1991; 325:1487–98.

4. Maillard SM, Jones R, Owens C, Pilkington C, Woo P, Wedderburn LR, et al. Quantitative assessment of MRI T2 relaxation time of thigh muscles in juvenile dermatomyositis. Rheumatology (Oxford). 2004; 43:603–8.

5. Connor A, Stebbings S, Anne Hung N, Hammond-Tooke G, Meikle G, Highton J. STIR MRI to direct muscle biopsy in suspected idiopathic inflammatory myopathy. J Clin Rheumatol. 2007; 13:341–5.

6. Walker UA. Imaging tools for the clinical assessment of idiopathic inflammatory myositis. Curr Opin Rheumatol. 2008; 20:656–61.

7. Adams EM, Chow CK, Premkumar A, Plotz PH. The idiopathic inflammatory myopathies: spectrum of MR imaging findings. Radiographics. 1995; 15:563–74.

8. O'Connell MJ, Powell T, Brennan D, Lynch T, McCarthy CJ, Eustace SJ. Whole-body MR imaging in the diagnosis of polymyositis. AJR Am J Roentgenol. 2002; 179:967–71.
9. Lundberg IE, Alexanderson H. Technology insight: tools for research, diagnosis and clinical assessment of treatment in idiopathic inflammatory myopathies. Nat Clin Pract Rheumatol. 2007; 3:282–90.

10. Tzaribachev N, Well C, Schedel J, Horger M. Whole-body MRI: a helpful diagnostic tool for juvenile dermatomyositis case report and review of the literature. Rheumatol Int. 2009; 29:1511–4.

11. Tomasová Studynková J, Charvát F, Jarosová K, Vencovsky J. The role of MRI in the assessment of polymyositis and dermatomyositis. Rheumatology (Oxford). 2007; 46:1174–9.
12. Compston A. Aids to the investigation of peripheral nerve injuries. Medical Research Council: Nerve Injuries Research Committee. His Majesty's Stationery Office:. 1942; 48. (iii) and 74 figures and 7 diagrams; with aids to the examination of the peripheral nervous system. By Michael O'Brien for the Guarantors of Brain. Saunders Elsevier: 2010; pp. [8] 64 and 94 Figures. Brain 2010; 133: 2838-44.
13. Lundberg I, Chung Y. Treatment and investigation of idiopathic inflammatory myopathies. Rheumatology (Oxford). 2000; 39:7–17.

14. Kimball AB, Summers RM, Turner M, Dugan EM, Hicks J, Miller FW, et al. Magnetic resonance imaging detection of occult skin and subcutaneous abnormalities in juvenile dermatomyositis. Implications for diagnosis and therapy. Arthritis Rheum. 2000; 43:1866–73.

15. Yoshida K, Kurosaka D, Joh K, Matsushima S, Takahashi E, Hirai K, et al. Fasciitis as a common lesion of dermatomyositis, demonstrated early after disease onset by en bloc biopsy combined with magnetic resonance imaging. Arthritis Rheum. 2010; 62:3751–9.

16. Grundtman C, Tham E, Ulfgren AK, Lundberg IE. Vascular endothelial growth factor is highly expressed in muscle tissue of patients with polymyositis and patients with dermatomyositis. Arthritis Rheum. 2008; 58:3224–38.

17. Chung YL, Wassif WS, Bell JD, Hurley M, Scott DL. Urinary levels of creatine and other metabolites in the assessment of polymyositis and dermatomyositis. Rheumatology (Oxford). 2003; 42:298–303.

18. Stonecipher MR, Jorizzo JL, White WL, Walker FO, Prichard E. Cutaneous changes of dermatomyositis in patients with normal muscle enzymes: dermatomyositis sine myositis? J Am Acad Dermatol. 1993; 28:951–6.

19. Bohan A, Peter JB, Bowman RL, Pearson CM. Computer-assisted analysis of 153 patients with polymyositis and dermatomyositis. Medicine (Baltimore). 1977; 56:255–86.
20. Olsen NJ, Qi J, Park JH. Imaging and skeletal muscle disease. Curr Rheumatol Rep. 2005; 7:106–14.

21. Kuo GP, Carrino JA. Skeletal muscle imaging and inflammatory myopathies. Curr Opin Rheumatol. 2007; 19:530–5.

Figure 1.
The signal intensity at STIR magnetic resonance imaging (MRI) of transaxial section. No signal intensity (arrowhead on Biceps femoris), subtle signal intensity (short narrow arrow on Semimembranous) were represented for score 0 and 1, respectively. Focal signal intensity (long narrow arrow on Vastus intermedialis) in each muscle which was affected less than 50% of area was represented for score 2. Diffuse signal intensity (wide arrow on Gracilis) was represented for score 3.
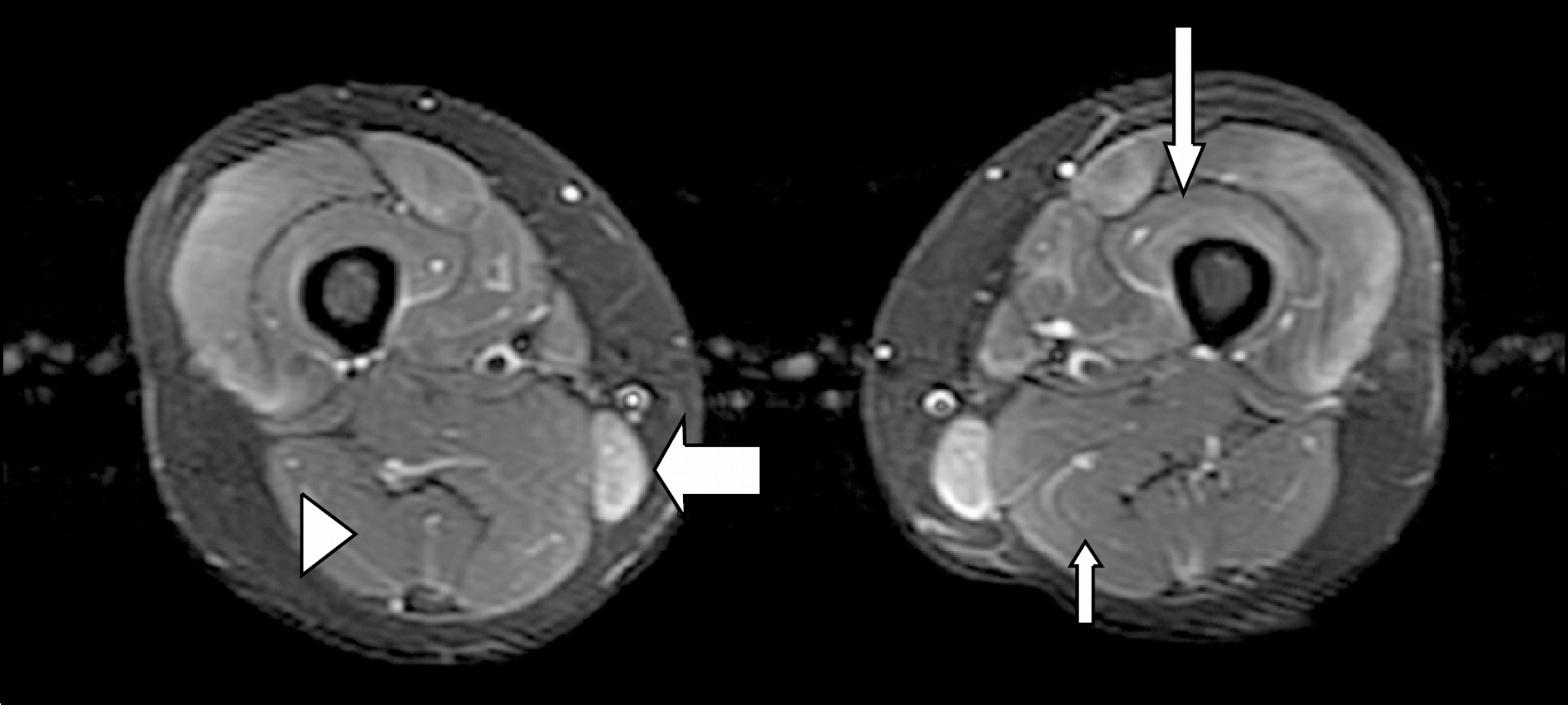
Figure 2.
Fasciitis on STIR magnetic resonance imaging of transaxial section in a dermatomyositis patient with myalgia symptom for 5 months until diagnosis. Areas of high signal intensity (arrows) observed in the fascias surrounding the Sartorius, Vastus intermedialis, Gracilis, Semimembranosus and Semitendinosus muscles.
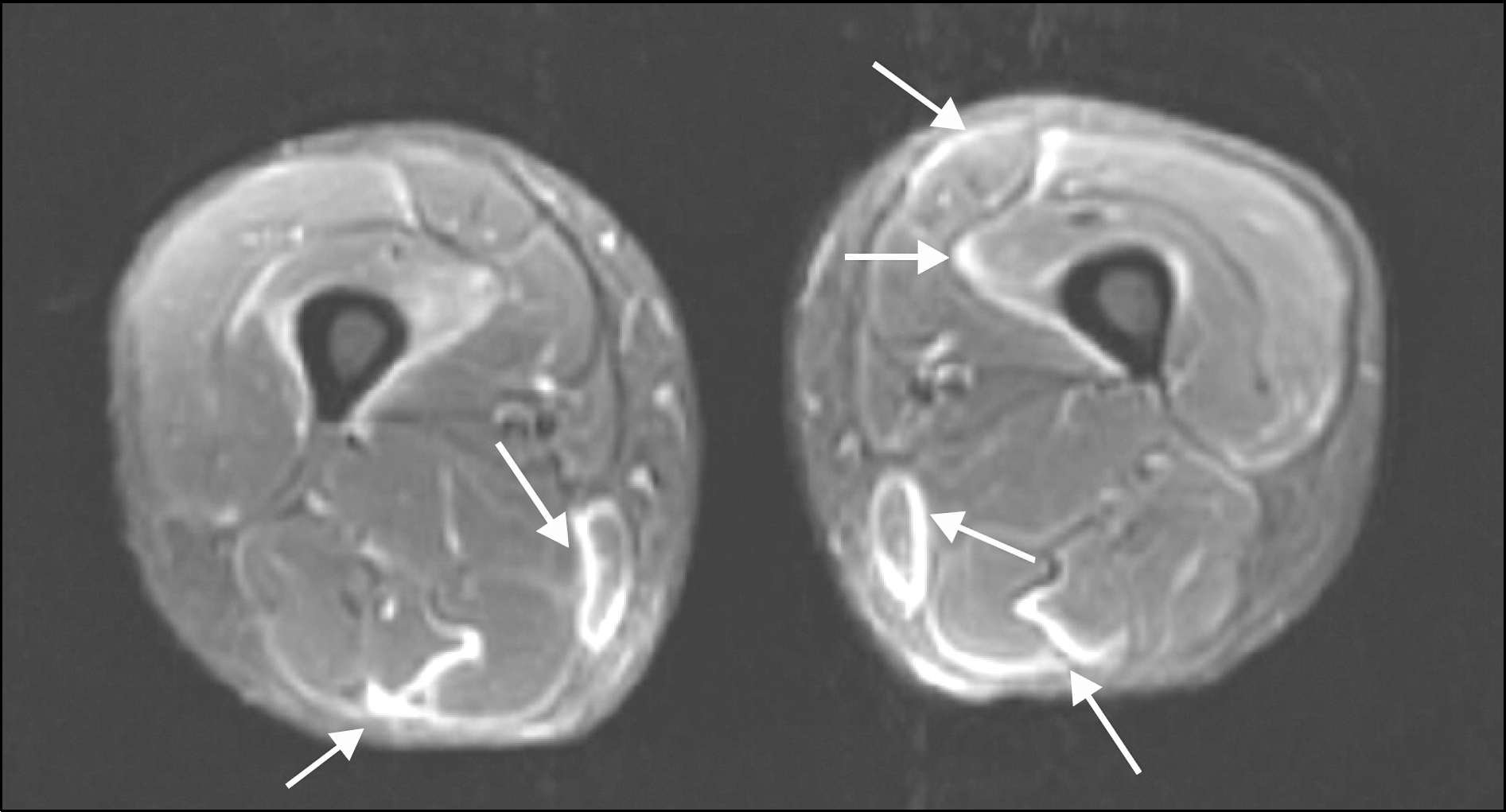
Figure 3.
Difference of total signal intensity depending on muscle power. Decreased muscle power MRC grade 0-IV, Normal muscle power MRC grade V.
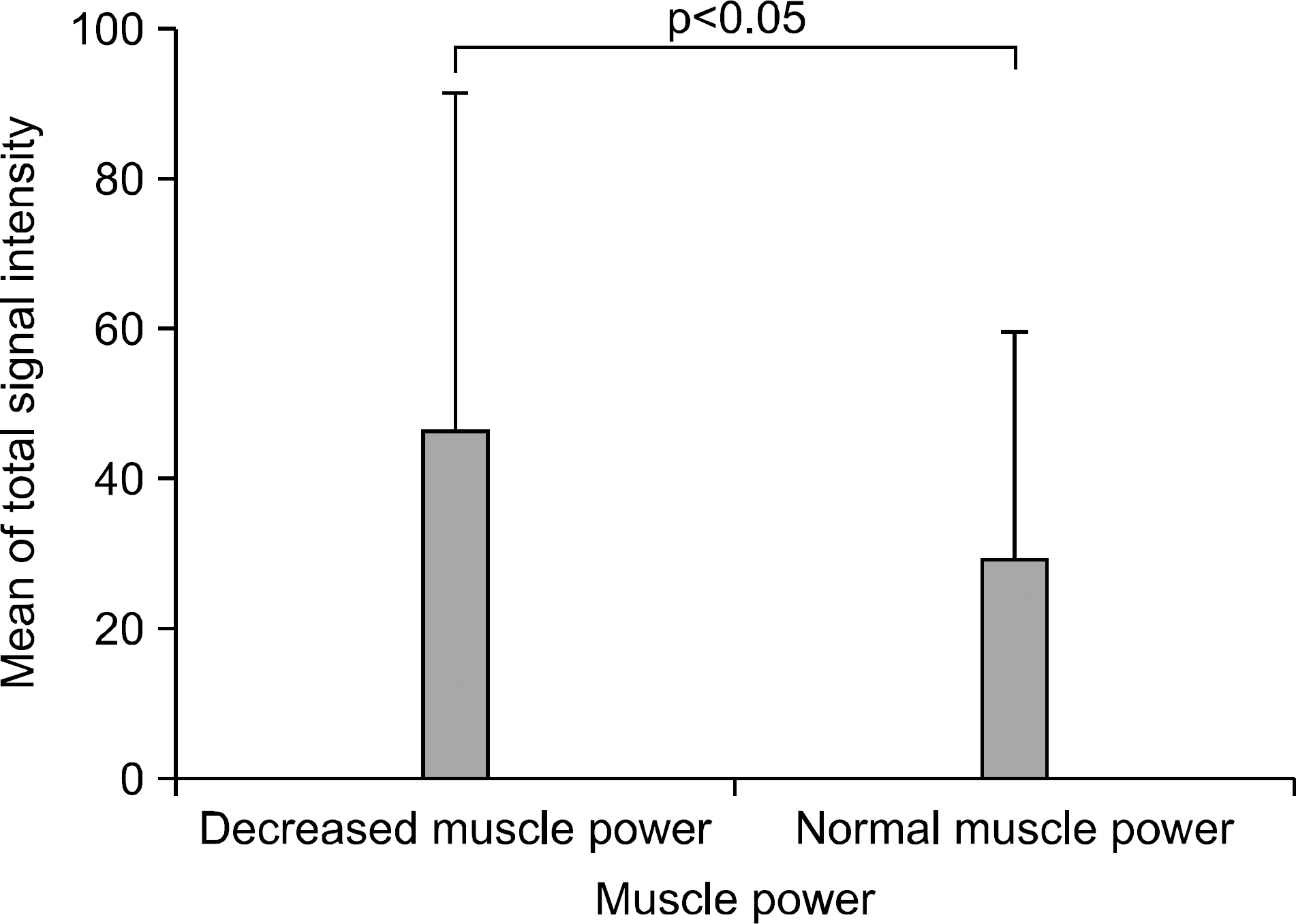
Figure 4.
Scatter plots of histopathologic scoring with MRI findings at biopsied muscles. Histopathologic scoring showed slightly positive correlations with signal intensity (r=0.339, p<0.05) (A) and edema portion (r=0.381, p<0.05) (B), and slightly negative correlation with atrophy portion (r=−0.311, p<0.05) (C) at the biopsied muscles.
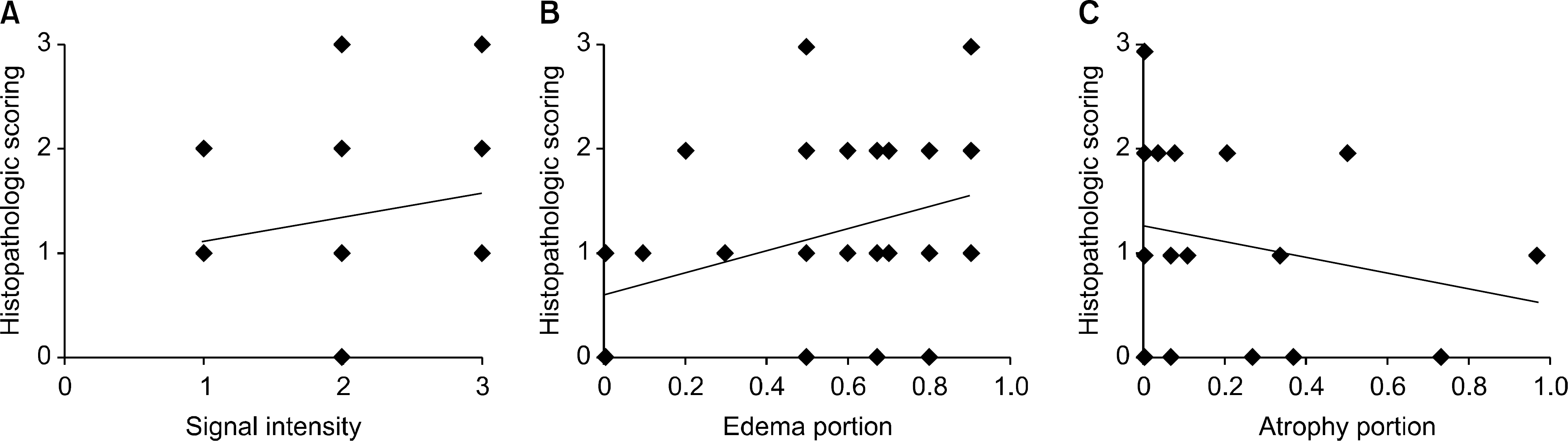
Table 1.
Demographic and clinical findings between patients with DM and PM
| DM (n=41) | PM (n=19) | Total (n=60) | |
|---|---|---|---|
| Age (years) at diagnosis | 41.9±13.0 | 40.2±14.6 | 41.4±13.4 |
| Female (%) | 80.5 | 84.2 | 81.7 |
| Duration† (months) | 5.2±5.2 | 9.7±13.1 | 6.6±8.7 |
| Muscle power scale | 4.0±0.8 | 3.9±0.6 | 3.9±0.8 |
| Myalgia (%) | 43.9 | 26.3 | 38.3 |
| AST (IU/L) | 79.1±70.0 | 110.5±113.7 | 89.0±86.3 |
| ALT (IU/L) | 64.2±64.5 | 93.37±82.42 | 73.4±71.3 |
| LDH (mg/dL) | 391.3±343.2 | 575.6±516.7 | 449.7±410.8 |
| CK (U/L)∗ | 976.0±1,547.2 | 2,520.7±3,450.2 | 1,465.2±2,404.1 |
| Aldolase (IU/mL)∗ | 17.6±16.5 | 33.2±33.4 | 22.5±24.1 |
| ESR (mm/hour) | 31.8±25.8 | 37.5±38.9 | 33.6±30.3 |
| CRP (mg/dL) | 0.7±1.0 | 0.5±0.5 | 0.6±0.9 |
| Anti-Jo1 positivity (%) | 7.3 | 6.3 | 7.0 |
| Malignancy (%) | 4.9 | 5.3 | 5.1 |
| Interstitial lung disease (%) | 70.3 | 53.8 | 66.0 |
Table 2.
MRI findings in patients with DM and PM
| DM (n=41) | PM (n=19) | Total (n=60) | |
|---|---|---|---|
| Symmetric involvement (%) | 39 (95.1) | 19 (100) | 58 (96.7) |
| TAM | 22.3±7.6 | 22.4±6.3 | 22.3±7.2 |
| TSI | 42.4±22.6 | 44.7±22.2 | 43.2±22.3 |
| Atrophy portion (%) | 11.1±22.7 | 20.2±31.8 | 14.0±26.0 |
| Fascia involvement (%)∗ | 23 (56.1) | 3 (15.8) | 26 (43.3) |
Table 3.
Correlation of MRI findings with laboratory findings




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download