Abstract
Objective
To compare the safety and efficacy associated with the addition of etanercept (ETN) with that of leflunomide (LEF) in Korean rheumatoid arthritis (RA) patients, who inadequately respond to methotrexate (MTX) in a randomized, open-label study.
Methods
Twenty-nine subjects suffering moderate to severe RA, despite MTX treatment were randomly assigned to a combination therapy with either ETN or LEF. The primary end-point was the proportion of subjects achieving American College of Rheumatology (ACR20) criteria at week 16.
Results
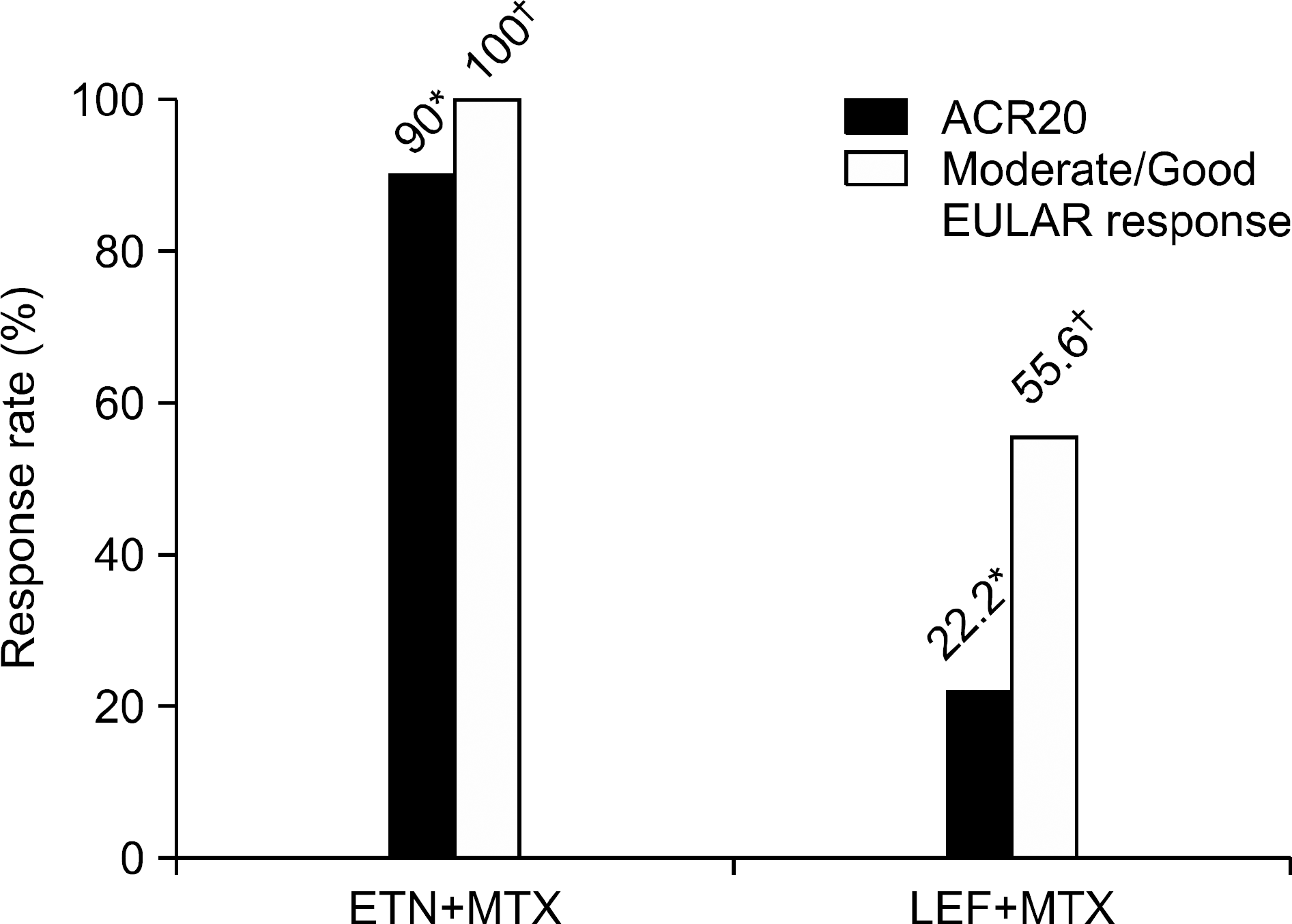
Ninety percent (n=18) of the ETN+MTX group (n=20) and 22.2% (n=2) of the LEF+MTX group (n=9) ach-ieved an ACR20 response (p=0.001). All patients (n=20) in the ETN+MTX group showed moderate or good EULAR response as compared with 55.6% (n=5) in the LEF+MTX group (p=0.012). All of the ETN+MTX subjects completed the study without adverse events. Adverse events occurred in 77.8% (n=7) of cases in the LEF+MTX group; sig-nificantly elevated serum AST/ALT levels in 6 subjects and mild neutropenia (ANC < 1,500/μ L) in 1 subject.
Conclusion
The ETN+MTX combination therapy was effective and safe, whereas the LEF+MTX combination therapy resulted in moderate efficacy in only half of the cases, and was accompanied by a high rate of adverse events. Elevated AST/ALT was the most common adverse event causing dose adjustment or discontinuation of therapeutic agent in the LEF+MTX group.
References
1. Bukhari MA, Wiles NJ, Lunt M, Harrison BJ, Scott DG, Symmons DP, et al. Influence of disease-modifying therapy on radiographic outcome in inflammatory polyarthritis at five years: results from a large observational in-ception study. Arthritis Rheum. 2003; 48:46–53.

2. Egsmose C, Lund B, Borg G, Pettersson H, Berg E, Brodin U, et al. Patients with rheumatoid arthritis benefit from early 2nd line therapy: 5 year followup of a prospective double blind placebo controlled study. J Rheumatol. 1995; 22:2208–13.
3. Saag KG, Teng GG, Patkar NM, Anuntiyo J, Finney C, Curtis JR, et al. American College of Rheumatology. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum. 2008; 59:762–84.

4. Emery P, Breedveld FC, Hall S, Durez P, Chang DJ, Robertson D, et al. Comparison of methotrexate mono-therapy with a combination of methotrexate and etanercept in active, early, moderate to severe rheumatoid arthritis (COMET): a randomised, double-blind, parallel treatment trial. Lancet. 2008; 372:375–82.

5. Cohen S, Cannon GW, Schiff M, Weaver A, Fox R, Olsen N, et al. Two-year, blinded, randomized, controlled trial of treatment of active rheumatoid arthritis with leflunomide compared with methotrexate. Utilization of Leflunomide in the Treatment of Rheumatoid Arthritis Trial Investigator Group. Arthritis Rheum. 2001; 44:1984–92.
6. Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988; 31:315–24.

7. Felson DT, Anderson JJ, Boers M, Bombardier C, Furst D, Goldsmith C, et al. American College of Rheumatology. Preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum. 1995; 38:727–35.

8. van Gestel AM, Prevoo ML, van't Hof MA, van Rijswijk MH, van de Putte LB, van Riel PL. Development and val-idation of the European League Against Rheumatism response criteria for rheumatoid arthritis. Comparison with the preliminary American College of Rheumatology and the World Health Organization/International League Against Rheumatism Criteria. Arthritis Rheum. 1996; 39:34–40.

9. Curtis JR, Beukelman T, Onofrei A, Cassell S, Greenberg JD, Kavanaugh A, et al. Elevated liver enzyme tests among patients with rheumatoid arthritis or psoriatic arthritis treated with methotrexate and/or leflunomide. Ann Rheum Dis. 2010; 69:43–7.

10. Lee SS, Park YW, Park JJ, Kang YM, Nam EJ, Kim SI, et al. Combination treatment with leflunomide and methotrexate for patients with active rheumatoid arthritis. Scand J Rheumatol. 2009; 38:11–4.

11. Alves JA, Fialho SC, Morato EF, Castro GR, Zimmer-mann AF, Ribeiro GG, et al. Liver toxicity is rare in rheumatoid arthritis patients using combination therapy with leflunomide and methotrexate. Rev Bras Reumatol. 2011; 51:141–4.
12. Kim HY, Hsu PN, Barba M, Sulaiman W, Robertson D, Vlahos B, et al. Randomized comparison of etanercept with usual therapy in an Asian population with active rheumatoid arthritis: the APPEAL trial. Int J Rheum Dis. 2012; 15:188–96.

13. Gutierrez-Ureña S, Molina JF, García CO, Cuéllar ML, Espinoza LR. Pancytopenia secondary to methotrexate therapy in rheumatoid arthritis. Arthritis Rheum. 1996; 39:272–6.

14. Keystone E, Haraoui B. Leflunomide. Hochberg MC, Silman AJ, Smolen JS, Weinblatt ME, Weisman MH, editors. Rheumatology. 5th ed.p. 519–27. Philadelphia, PA: Mosby/Elsevier;2011.
Figure 1.
Patient disposition. LEF: lefunomide, MTX: methotrexate, ETN: etanercept, LFT: liver function test, ACR: American College of Rheumatology, EULAR: European League Against Rheumatism. ∗p=0.001, † p=0.012, ‡ p<0.001. Fisher's exact test.
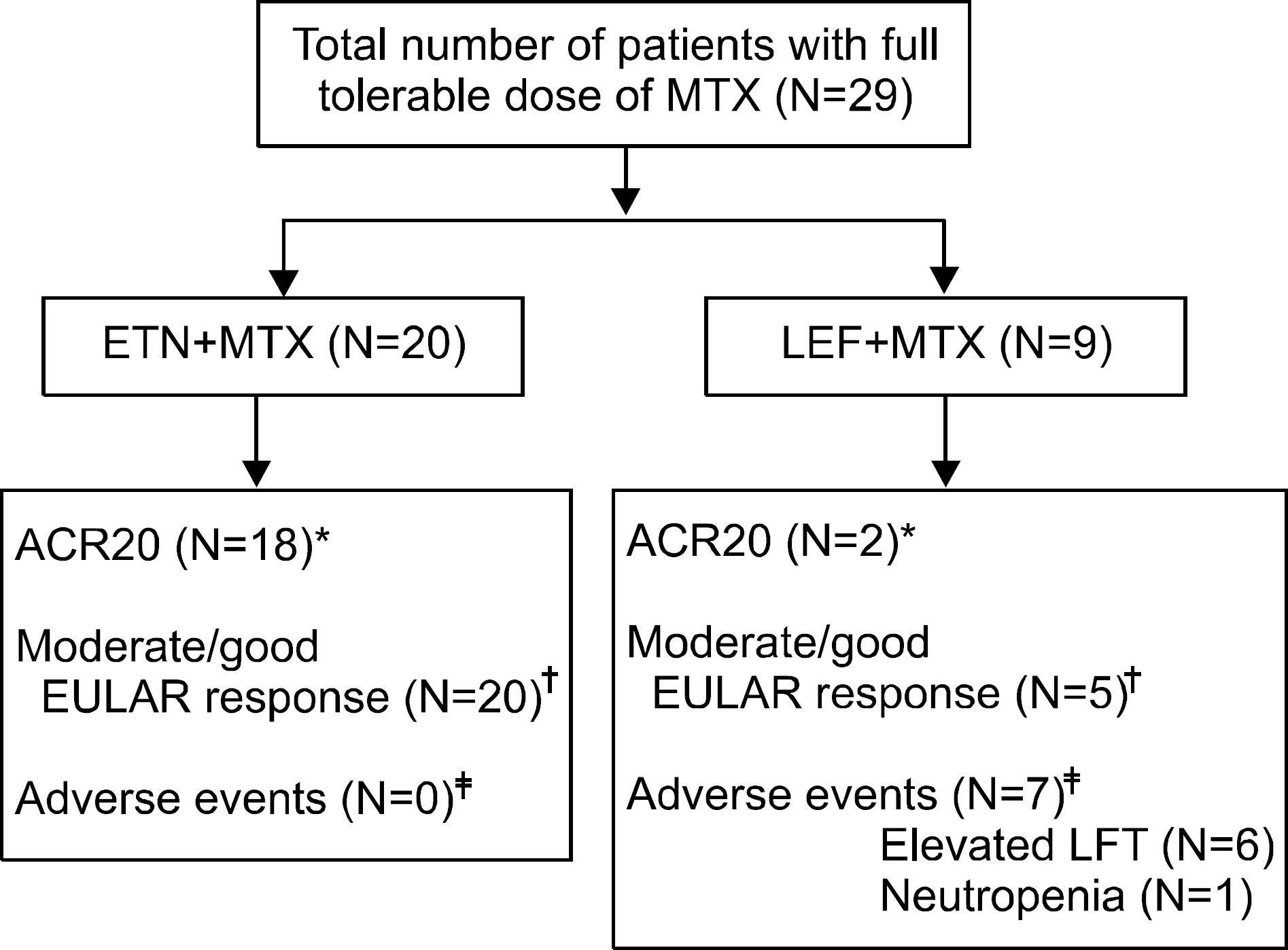
Table 1.
Baseline demographics and characteristics of both patient groups.
All values are mean± SD unless otherwise specified. LEF: lefunomide, MTX: methotrexate, ETN: etanercept, BMI: body mass index, DM: diabetes mellitus, NSAIDS: nonsteroidal antiinflammatory drugs, INH: isoniazid, DAS28: disease activity score based on a 28-joint count, ESR: erythrocyte sedimentation rate. The Mann-Whitney U test was used to compare the variables
Table 2.
Characteristics of patients in the LEF+MTX treatment group




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download