Abstract
Objectives
To investigate the perception of and treatment pattern with regard to the four important issues in the management of lupus nephritis (LN), and to identify which parts of the LN treatment are difficult for physicians to carry out in clinical practice.
Methods
Four steps were carried out: pre-survey, LN symposium, post-survey, and meeting after the symposium. The two surveys were conducted with the same contents regarding renal biopsy, induction and maintenance treatment for class III and IV LN, and treatment for class V LN. The results of the first survey and the changes in opinion re-flected in the second survey were comparatively analyzed.
Results
In the first survey, most of the respondent physicians replied that they would immediately conduct biopsy in the case of significant proteinuria. For the induction treatment of class III and IV LN, most of the respondent physicians selected high-dose cyclophosphamide. Mycophenolate mofetil and steroid combination therapy were selected for the maintenance treatment, and tacrolimus for the treatment of class V LN. There was a controversy in the drug selection, however, especially on the maintenance treatment of class III and IV LN and on the treatment of non-responsive class V LN.
Conclusion
Some discrepancies were found in the treatment of LN in the real world. Although no recommendation was made for Korean LN patients in this study, the study results will help physicians select the most rea-sonable treatment for Korean LN patients based on ex-perts’ experiences and objective evidence.
Go to : 
References
2. Cameron JS. Lupus nephritis: an historical perspective 1968–1998. J Nephrol. 1999; 12(Suppl 2):S29–41.
3. Bernatsky S, Boivin JF, Joseph L, Manzi S, Ginzler E, Gladman DD, et al. Mortality in systemic lupus erythematosus. Arthritis Rheum. 2006; 54:2550–7.

4. Norby GE, Lerang K, Holdaas H, Gran JT, Str⊘m EH, Draganov B, et al. Lupus-nephritis–diagnosis and treatment. Tidsskr Nor Laegeforen. 2010; 130:1140–4.
5. Weening JJ, D'Agati VD, Schwartz MM, Seshan SV, Alpers CE, Appel GB, et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. J Am Soc Nephrol. 2004; 15:241–50.

6. Hahn BH, McMahon MA, Wilkinson A, Wallace WD, Daikh DI, Fitzgerald JD, et al. American College of Rheumatology. American College of Rheumatology guidelines for screening, treatment, and management of lupus nephritis. Arthritis Care Res (Hoboken). 2012; 64:797–808.

7. Bertsias GK, Tektonidou M, Amoura Z, Aringer M, Bajema I, Berden JH, et al. European League Against Rheumatism and European Renal Association-European Dialysis and Transplant Association. Joint European League Against Rheumatism and European Renal Association-European Dialysis and Transplant Association (EULAR/ERA-EDTA) recommendations for the management of adult and paediatric lupus nephritis. Ann Rheum Dis. 2012; 71:1771–82.

8. van Tellingen A, Voskuyl AE, Vervloet MG, Bijl M, de Sévaux RG, Berger SP, et al. Dutch Working Party on Systemic Lupus Erythematosus. Dutch guidelines for diagnosis and therapy of proliferative lupus nephritis. Neth J Med. 2012; 70:199–207.
9. Appel GB, Contreras G, Dooley MA, Ginzler EM, Isenberg D, Jayne D, et al. Aspreva Lupus Management Study Group. Mycophenolate mofetil versus cyclophosphamide for induction treatment of lupus nephritis. J Am Soc Nephrol. 2009; 20:1103–12.

10. Chan TM, Tse KC, Tang CS, Mok MY, Li FK. Hong Kong Nephrology Study Group. Longterm study of mycophenolate mofetil as continuous induction and maintenance treatment for diffuse proliferative lupus nephritis. J Am Soc Nephrol. 2005; 16:1076–84.

11. Ginzler EM, Dooley MA, Aranow C, Kim MY, Buyon J, Merrill JT, et al. Mycophenolate mofetil or intravenous cyclophosphamide for lupus nephritis. N Engl J Med. 2005; 353:2219–28.

12. Ong LM, Hooi LS, Lim TO, Goh BL, Ahmad G, Ghazalli R, et al. Randomized controlled trial of pulse intravenous cyclophosphamide versus mycophenolate mofetil in the induction therapy of proliferative lupus nephritis. Nephrology (Carlton). 2005; 10:504–10.

13. Touma Z, Gladman DD, Urowitz MB, Beyene J, Uleryk EM, Shah PS. Mycophenolate mofetil for induction treatment of lupus nephritis: a systematic review and meta-analysis. J Rheumatol. 2011; 38:69–78.

14. Dooley MA, Jayne D, Ginzler EM, Isenberg D, Olsen NJ, Wofsy D, et al. ALMS Group. Mycophenolate versus azathioprine as maintenance therapy for lupus nephritis. N Engl J Med. 2011; 365:1886–95.

15. Illei GG, Austin HA, Crane M, Collins L, Gourley MF, Yarboro CH, et al. Combination therapy with pulse cyclophosphamide plus pulse methylprednisolone improves longterm renal outcome without adding toxicity in patients with lupus nephritis. Ann Intern Med. 2001; 135:248–57.

16. Boumpas DT, Austin HA 3rd, Vaughan EM, Yarboro CH, Klippel JH, Balow JE. Risk for sustained amenorrhea in patients with systemic lupus erythematosus receiving in-termittent pulse cyclophosphamide therapy. Ann Intern Med. 1993; 119:366–9.

17. Houssiau FA, D'Cruz D, Sangle S, Remy P, Vasconcelos C, Petrovic R, et al. MAINTAIN Nephritis Trial Group. Azathioprine versus mycophenolate mofetil for longterm immunosuppression in lupus nephritis: results from the MAINTAIN Nephritis Trial. Ann Rheum Dis. 2010; 69:2083–9.

18. Austin HA 3rd, Illei GG, Braun MJ, Balow JE. Randomized, controlled trial of prednisone, cyclophosphamide, and cyclosporine in lupus membranous nep-hropathy. J Am Soc Nephrol. 2009; 20:901–11.

19. Moroni G, Doria A, Mosca M, Alberighi OD, Ferraccioli G, Todesco S, et al. A randomized pilot trial comparing cyclosporine and azathioprine for maintenance therapy in diffuse lupus nephritis over four years. Clin J Am Soc Nephrol. 2006; 1:925–32.

20. Ogawa H, Kameda H, Nagasawa H, Sekiguchi N, Takei H, Tsuzaka K, et al. Prospective study of low-dose cyclosporine A in patients with refractory lupus nephritis. Mod Rheumatol. 2007; 17:92–7.

21. Lee YH, Woo JH, Choi SJ, Ji JD, Song GG. Induction and maintenance therapy for lupus nephritis: a systematic review and meta-analysis. Lupus. 2010; 19:703–10.

22. Lee YH, Lee HS, Choi SJ, Dai Ji J, Song GG. Efficacy and safety of tacrolimus therapy for lupus nephritis: a systematic review of clinical trials. Lupus. 2011; 20:636–40.

Go to : 
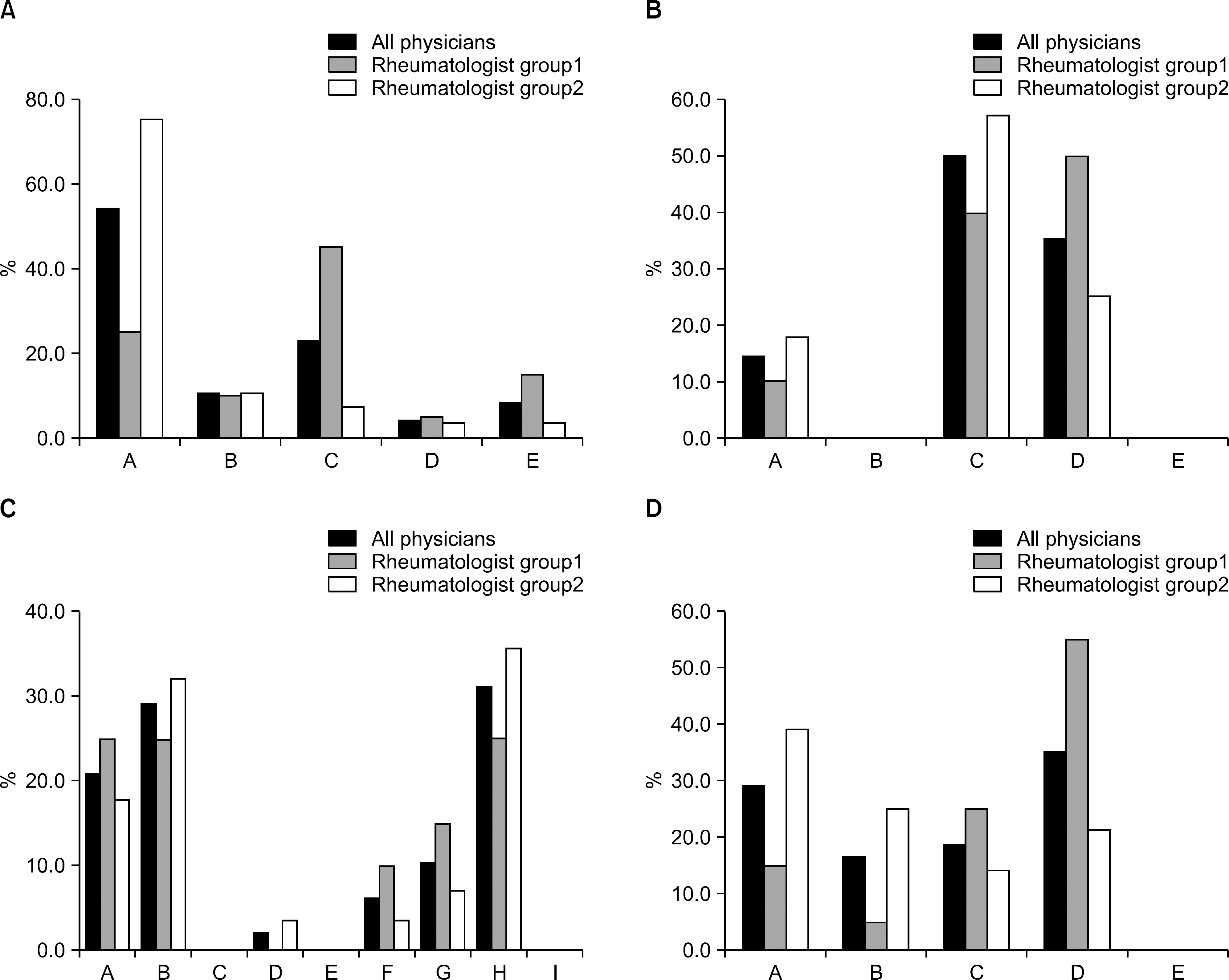 | Figure 2.(A) First-survey results on the diagnostic performance of renal biopsy. A: Immediate conduct of renal biopsy. B: Renal biopsy when the steroid dose is decreased to 20 mg/day or lower. C: Immunosuppressant administration, but if no response, renal biopsy. D: Biopsy omitted. E: Others. (B) First-survey results on the induction therapy on LN. A: Oral administration of mycophenolatemofetil. B: Oral administration of tacrolimus. C: Intravenous cyclophosphamide administration (NIH protocol). D: Intravenous cyclophosphamide administration (Eurolupus protocol). E: Others. (C) First-survey results on the maintenance therapy on LN. A: Azathioprine. B: Azathioprine+ glucocorticoid. C: Cyclosporine. D: Cyclosporine+ glucocorticoid. E: Cyclophosphamide. F: Cyclophosphamide+ glucocorticoid. G: Mycophenolatemofetil. H: Mycophenolatemofetil+ glucocorticoid. I: Others. (D) First-survey results on the maintenance therapy on LN. A: Maintenance and observation of the current treatment. B: Cyclophosphamide IV administration. C: Oral administration of mycophenolatemofetil. D: Oral administration of tacrolimus. E: Others. |
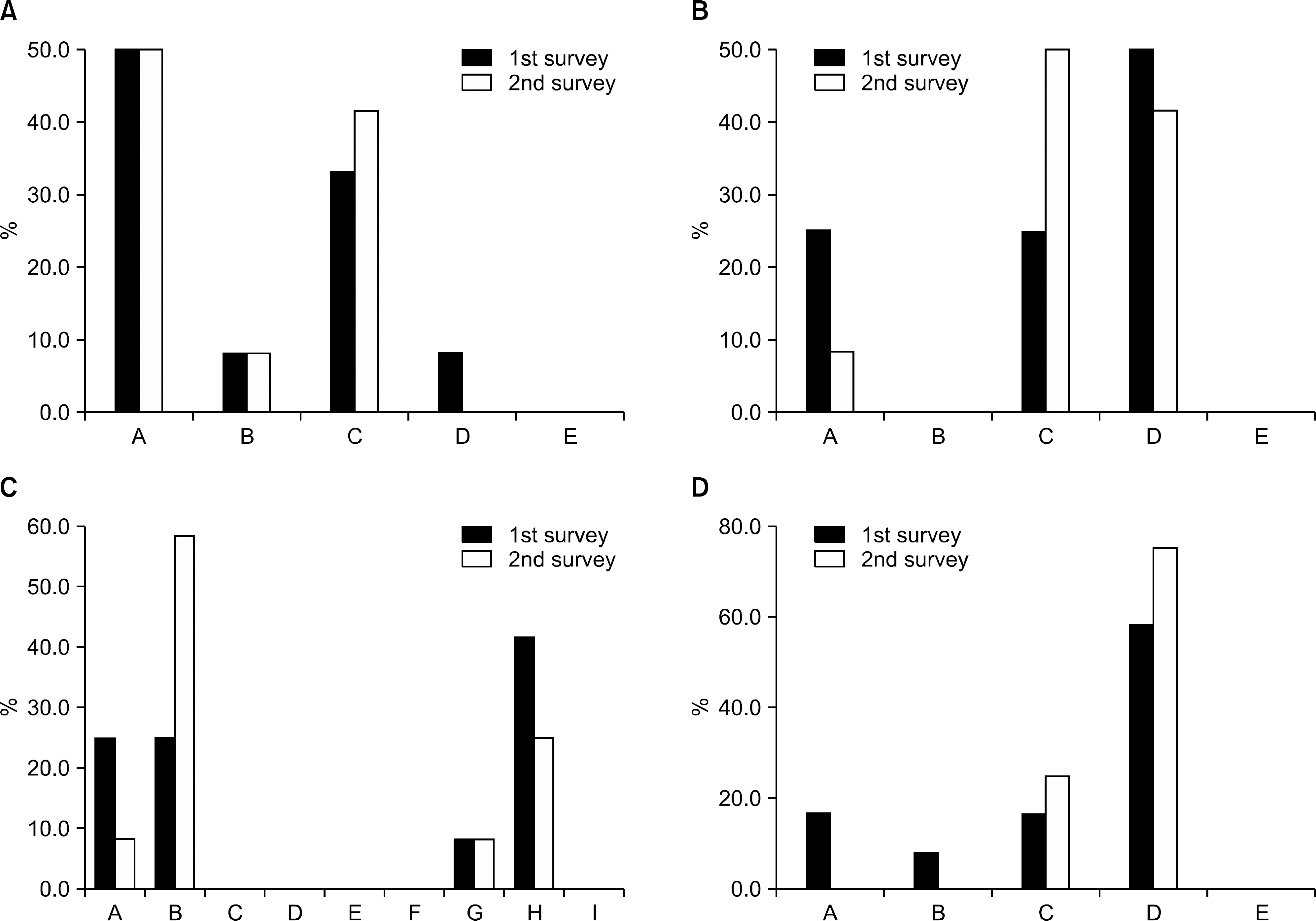 | Figure 3.(A) Change of opinion on the diagnostic performance of renal biopsy. A: Immediate conduct of renal biopsy. B: Renal biopsy when the steroid dose is decreased to 20 mg/day or lower. C: Immunosuppressant administration, but if no response, renal biopsy. D: Biopsy omitted. E: Others. (B) Change of opinion on the induction therapy on LN. A: Oral administration of mycophenolatemofetil. B: Oral administration of tacrolimus. C: Intravenous cyclophosphamide administration (NIH protocol). D: Intravenous cyclophosphamide administration (Eurolupus protocol). E: Others. (C) Change of opinion on the maintenance therapy on LN. A: Azathioprine. B: Azathioprine+ glucocorticoid. C: Cyclosporine. D: Cyclosporine+ glucocorticoid. E: Cyclophosphamide. F: Cyclophosphamide+ glucocorticoid. G: Mycophenolatemofetil. H: Mycophenolatemofetil+ glucocorticoid. I: Others. (D) Change of opinion on the maintenance therapy on LN. A: Maintenance and observation of the current treatment. B: Cyclophosphamide IV administration. C: Oral administration of mycophenolatemofetil. D: Oral administration of tacrolimus. E: Others. |
Table 1.
Baseline characteristics of the physicians who participated in the first survey
Table 2.
Clinical scenarios and questionnaires of the surveys conducted before and after the symposium
Table 3.
Responses to the four scenarios according to the length of experience in taking care of SLE patients




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download