Abstract
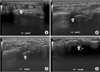
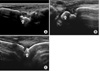
Bruton-type agammaglobulinemia is primary hypogammaglobulinemia followed by severe recurrent infection, including bacterial otitis media, bronchitis, pneumonia, and meningitis. Septic arthritis is a main musculoskeletal disorder that can occur in association with Bruton-type agammaglobulinemia. But the development of rheumatoid arthritis (RA) is rarely reported in a patient with hypogammaglobulinemia. Here, we describe a case of 34-year-old male with Bruton-type agammaglobulinemia, who presented with multiple symmetric polyarthritis. He was diagnosed as having a RA according to ACR criteria. His symptoms of polyarthritis had been improved after the introduction of medications including DMARDs (disease modifying anti-rheumatic drugs). Our case suggests that RA can be developed in the setting of agammaglobulinemia, and even in this situation, anti-rheumatic agents were effective to control arthritis without complication such as severe infection.
Figures and Tables
References
1. Bruton OC. Agammaglobulinemia. Pediatrics. 1952. 9:722–728.
2. Conley ME. Molecular approaches to analysis of X-linked immunodeficiencies. Annu Rev Immunol. 1992. 10:215–238.
3. Väliaho J, Smith CI, Vihinen M. BTKbase: the mutation database for X-linked agammaglobulinemia. Hum Mutat. 2006. 27:1209–1217.
4. Kim YC, Choi I, Lee M, Goh YI, Jung IJ, Park KO, et al. A Case of Bruton's Agammaglobulinemia. Korean J Intern Med. 1994. 47:721–726.
5. Lee AH, Levinson AI, Schumacher HR Jr. Hypogammaglobulinemia and rheumatic disease. Semin Arthritis Rheum. 1993. 22:252–264.
6. Swaak AJ, van den Brink HG. Common variable immunodeficiency in a patient with systemic lupus erythematosus. Lupus. 1996. 5:242–246.
7. Munthe E, Hoyeraal HM, Froland SS, Mellbye OJ, Kåss E, Natvig JB. Evidence for complement activation by the alternate pathway in the arthritis of hypogammaglobulinemic patients. Rheumatology. 1975. 6:43–51.
8. Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988. 31:315–324.
9. Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010. 62:2569–2581.
10. Sany J, Jorgensen CH, Anaya JM, Didry C, Andary M, Serre I, et al. Arthritis associated with primary agammaglobulinemia: new clues to its immunopathology. Clin Exp Rheumatol. 1993. 11:65–69.
11. Hansel TT, Haeney MR, Thompson RA. Primary hypogammaglobulinaemia and arthritis. Br Med J (Clin Res Ed). 1987. 295:174–175.
12. Verbruggen G, De Backer S, Deforce D, Demetter P, Cuvelier C, Veys E, et al. X linked agammaglobulinaemia and rheumatoid arthritis. Ann Rheum Dis. 2005. 64:1075–1078.
13. Pipitone N, Jolliffe VA, Cauli A, Scott DG, Pitzalis C. Do B cells influence disease progression in chronic synovitis? Lessons from primary hypogammaglobulinaemia. Rheumatology (Oxford). 2000. 39:1280–1285.
14. Chattopadhyay C, Chattopadhyay H, Natvig JB, Michaelsen TE, Mellbye OJ. Lack of suppressor cell activity in rheumatoid synovial lymphocytes. Scand J Immunol. 1979. 10:309–316.
15. Cooper MD, Keightley RG, Webb SR. T- and B-cell interactions in autoimmune syndromes. Ann N Y Acad Sci. 1975. 256:105–116.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download