Abstract
Objective
Angiopoietin-1 (Ang1) is a potent angiogenic factor that can increase synovial angiogenesis and also en-hance osteoblast maturation and bone formation. However, its role in rheumatoid arthritis (RA) has not been well documented. Thus, we investigated roles of Ang1 in collagen-induced arthritis (CIA).
Methods
A recombinant adenovirus carrying the gene that encodes either cartilage oligomeric matrix protein (AdCOMP)-Ang1 (a modified form of Ang1) or LacZ (AdLacZ) was injected intravenously into CIA mice. Clinical, radiological, histopathological, and immuno-fluorescent analyses were performed. Serum levels of receptor activators of nuclear factor κ B ligand (RANKL) and osteoprotegerin (OPG) and expression of osteoblast maturation genes were analyzed.
Results
AdCOMP-Ang1-injected mice developed more severe inflammation than the AdLacZ-injected mice. However, there were no significant differences in cartilage damage and bone erosion. More PECAM-1-positive blood vessels were seen in the synovium of the AdCOMP-Ang1-injected mice than in those injected with AdLacZ. Interestingly, a lower number of TRAP-positive osteoclasts were observed in AdCOMP-Ang1-injected CIA mice than in the AdLacZ group when comparing sections obtained from joints showing similar synovial proliferation. The serum OPG/RANKL ratio and expression of osteoblast maturation genes, such as runt-related transcription factor 2, bone sialoprotein, type 1 collagen, osteopontin, and osterix, were significantly upregulated in the AdCOMP-Ang1 group.
Conclusion
COMP-Ang1 facilitates arthritis onset and increases synovial inflammation, but enhances osteoblast maturation, which in turn inhibits osteoclastogenesis by increasing the OPG/RANKL ratio in CIA. Our results sug-gest that careful investigation is necessary to delineate the possible therapeutic use of COMP-Ang1 as an adjunctive agent, in combination with antiinflammatory therapies, for the prevention of bone destruction in RA.
Go to : 
References
2. Lainer-Carr D, Brahn E. Angiogenesis inhibition as a therapeutic approach for inflammatory synovitis. Nat Clin Pract Rheumatol. 2007; 3:434–42.

3. Szekanecz Z, Koch AE. Mechanisms of Disease: angiogenesis in inflammatory diseases. Nat Clin Pract Rheumatol. 2007; 3:635–43.

4. Jones N, Iljin K, Dumont DJ, Alitalo K. Tie receptors: new modulators of angiogenic and lymphangiogenic responses. Nat Rev Mol Cell Biol. 2001; 2:257–67.

5. Augustin HG, Koh GY, Thurston G, Alitalo K. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat Rev Mol Cell Biol. 2009; 10:165–77.

6. DeBusk LM, Chen Y, Nishishita T, Chen J, Thomas JW, Lin PC. Tie2 receptor tyrosine kinase, a major mediator of tumor necrosis factor alpha-induced angiogenesis in rheumatoid arthritis. Arthritis Rheum. 2003; 48:2461–71.
7. Gravallese EM, Pettit AR, Lee R, Madore R, Manning C, Tsay A, et al. Angiopoietin-1 is expressed in the synovium of patients with rheumatoid arthritis and is induced by tu-mour necrosis factor α. Ann Rheum Dis. 2003; 62:100–7.
8. Chen Y, Donnelly E, Kobayashi H, Debusk LM, Lin PC. Gene therapy targeting the Tie2 function ameliorates col-lagen-induced arthritis and protects against bone destruction. Arthritis Rheum. 2005; 52:1585–94.

9. Romas E, Gillespie MT, Martin TJ. Involvement of receptor activator of NFkappaB ligand and tumor necrosis factor-α in bone destruction in rheumatoid arthritis. Bone. 2002; 30:340–6.
10. Takayanagi H. Osteoimmunology and the effects of the immune system on bone. Nat Rev Rheumatol. 2009; 5:667–76.

11. Choi Y, Arron JR, Townsend MJ. Promising bone-related therapeutic targets for rheumatoid arthritis. Nat Rev Rheumatol. 2009; 5:543–8.

12. Glass DA 2nd, Bialek P, Ahn JD, Starbuck M, Patel MS, Clevers H, et al. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell. 2005; 8:751–64.

13. Walsh NC, Reinwald S, Manning CA, Condon KW, Iwata K, Burr DB, et al. Osteoblast function is compromised at sites of focal bone erosion in inflammatory arthritis. J Bone Miner Res. 2009; 24:1572–85.

14. Pap T, Distler O. Linking angiogenesis to bone destruction in arthritis. Arthritis Rheum. 2005; 52:1346–8.

15. Suzuki T, Miyamoto T, Fujita N, Ninomiya K, Iwasaki R, Toyama Y, et al. Osteoblast-specific Angiopoietin 1 overexpression increases bone mass. Biochem Biophys Res Commun. 2007; 362:1019–25.

16. Cho CH, Kammerer RA, Lee HJ, Steinmetz MO, Ryu YS, Lee SH, et al. COMP-Ang1: a designed angiopoie-tin-1 variant with nonleaky angiogenic activity. Proc Natl Acad Sci U S A. 2004; 101:5547–52.

17. Cho CH, Kim KE, Byun J, Jang HS, Kim DK, Baluk P, et al. Longterm and sustained COMP-Ang1 induces long-lasting vascular enlargement and enhanced blood flow. Circ Res. 2005; 97:86–94.

18. Thurston G, Suri C, Smith K, McClain J, Sato TN, Yancopoulos GD, et al. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science. 1999; 286:2511–4.

19. Jeong BC, Kim HJ, Bae IH, Lee KN, Lee KY, Oh WM, et al. COMP-Ang1, a chimeric form of Angiopoietin 1, enhances BMP2-induced osteoblast differentiation and bone formation. Bone. 2010; 46:479–86.

20. Park BH, Jang KY, Kim KH, Song KH, Lee SY, Yoon SJ, et al. COMP-Angiopoietin-1 ameliorates surgery-induced ischemic necrosis of the femoral head in rats. Bone. 2009; 44:886–92.

21. Park BH, Yoon SJ, Jang KY, Kim MR, Lee HS, Kim KB, et al. COMP-angiopoietin-1 accelerates bone formation during distraction osteogenesis. Bone. 2010; 46:1442–8.

22. Hah YS, Lee YR, Jun JS, Lim HS, Kim HO, Jeong YG, et al. A20 suppresses inflammatory responses and bone destruction in human fibroblast-like synoviocytes and in mice with collagen-induced arthritis. Arthritis Rheum. 2010; 62:2313–21.

23. Lee S, Kim W, Kim DH, Moon SO, Jung YJ, Lee AS, et al. Protective effect of COMP-angiopoietin-1 on cyclosporine-induced renal injury in mice. Nephrol Dial Transplant. 2008; 23:2784–94.

24. Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov. 2007; 6:273–86.

26. Eskens FA, Verweij J. The clinical toxicity profile of vascular endothelial growth factor (VEGF) and vascular endothelial growth factor receptor (VEGFR) targeting angiogenesis inhibitors; a review. Eur J Cancer. 2006; 42:3127–39.

27. Hawighorst T, Skobe M, Streit M, Hong YK, Velasco P, Brown LF, et al. Activation of the tie2 receptor by angio-poietin-1 enhances tumor vessel maturation and impairs squamous cell carcinoma growth. Am J Pathol. 2002; 160:1381–92.

28. Machein MR, Knedla A, Knoth R, Wagner S, Neuschl E, Plate KH. Angiopoietin-1 promotes tumor angiogenesis in a rat glioma model. Am J Pathol. 2004; 165:1557–70.

29. Voskas D, Jones N, Van Slyke P, Sturk C, Chang W, Haninec A, et al. A cyclosporine-sensitive psoriasis-like disease produced in Tie2 transgenic mice. Am J Pathol. 2005; 166:843–55.

30. Hashiramoto A, Sakai C, Yoshida K, Tsumiyama K, Miura Y, Shiozawa K, et al. Angiopoietin 1 directly induces destruction of the rheumatoid joint by cooperative, but independent, signaling via ERK/MAPK and phosphati-dylinositol 3-kinase/Akt. Arthritis Rheum. 2007; 56:2170–9.

31. Komori T. Regulation of skeletal development by the Runx family of transcription factors. J Cell Biochem. 2005; 95:445–53.

Go to : 
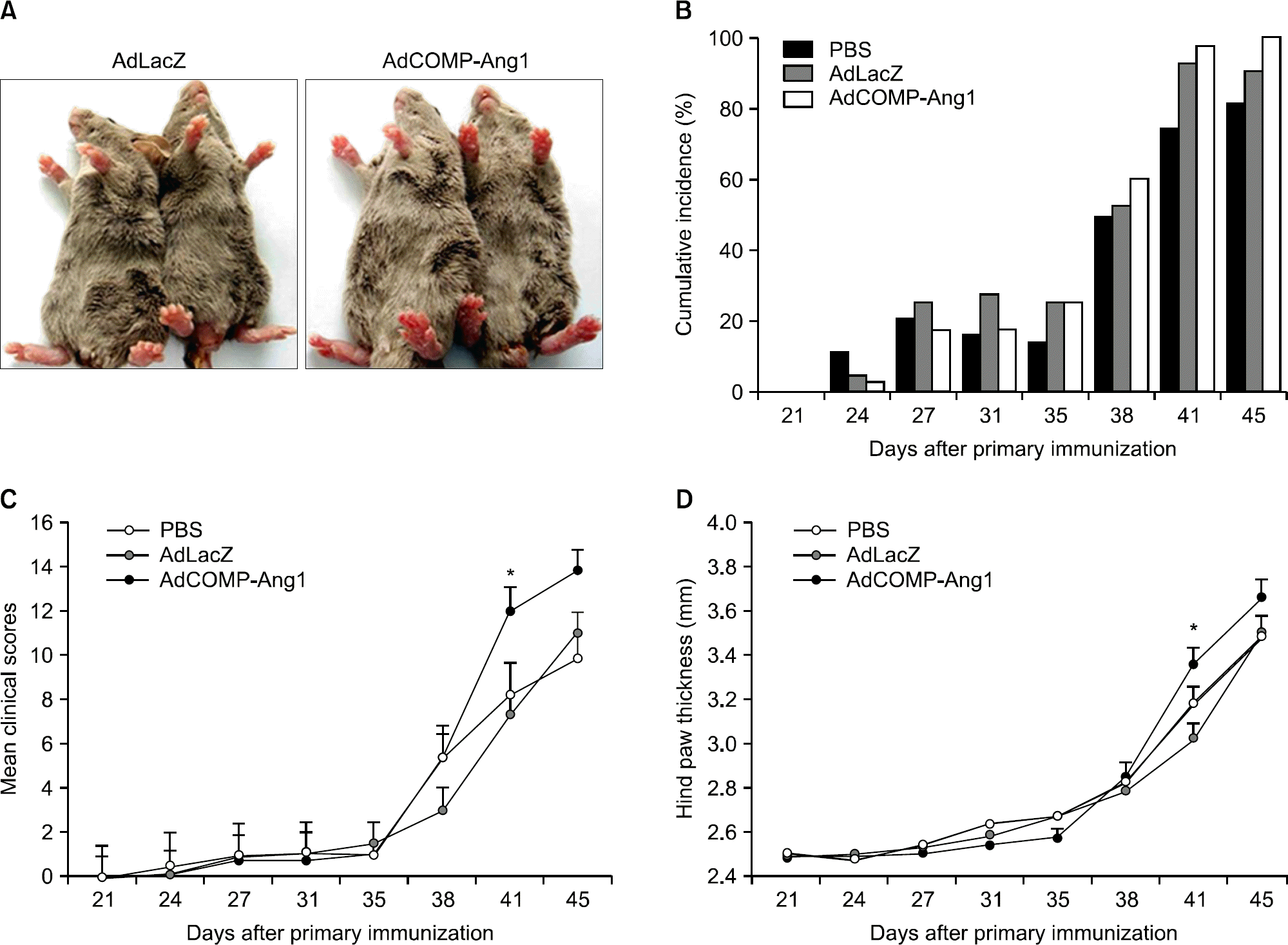 | Figure 1.COMP-Ang1 increases erythema and joint swelling. (A) Representative photographs showing the gross features of the hind paws. The mouse injected with AdCOMP-Ang1 shows increased redness (right panel) compared with the Ad-LacZ mouse (left panel), despite showing comparable arthritic development in all paws. (B) The cumulative incidence of arthritis, (C) severity of arthritis, as assessed by a visual arthritis scoring system and (D) hind paw thickness were determined (n=10 for each group). Values are the mean± SEM, ∗p<0.05 vs. AdLacZ. |
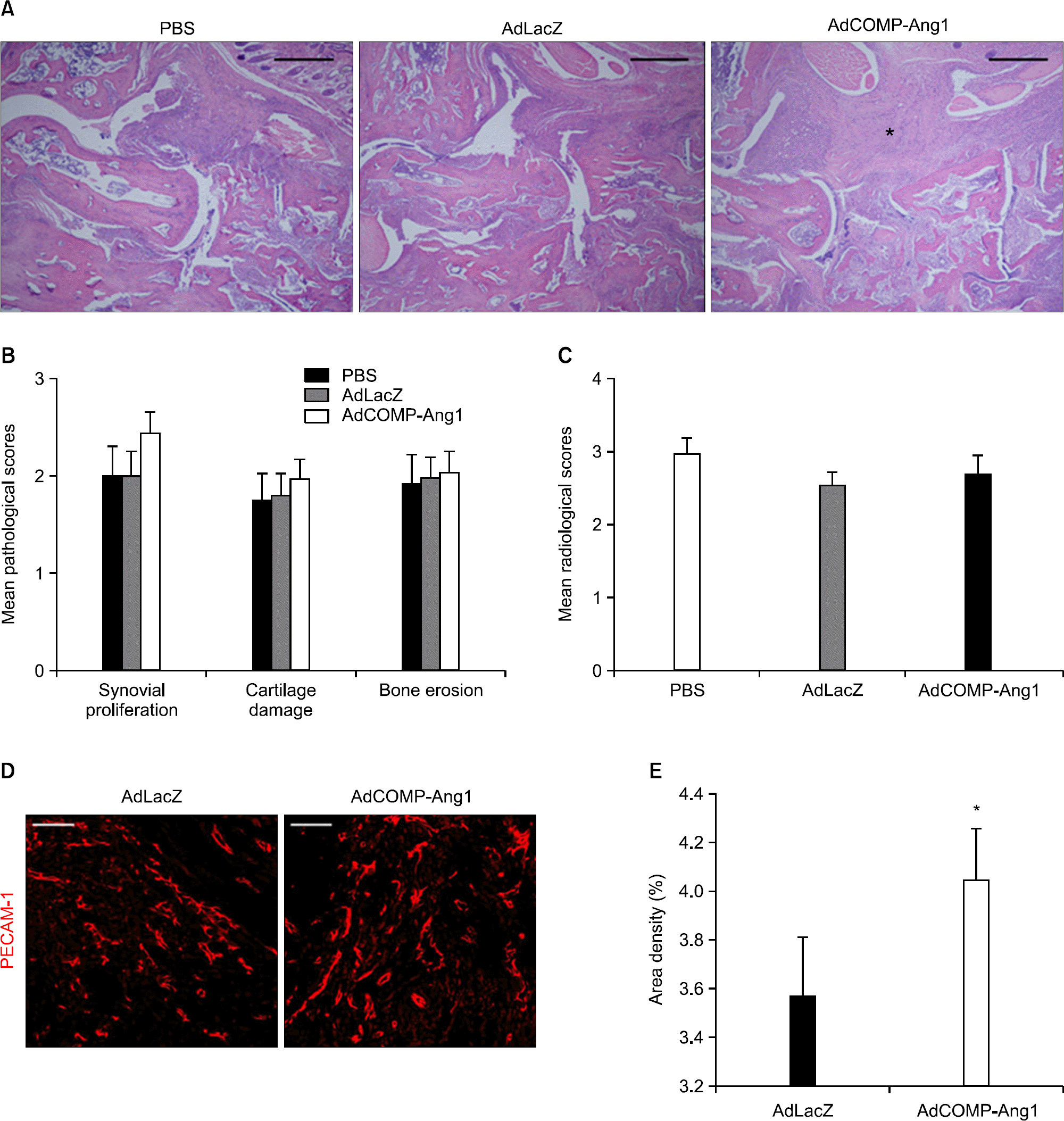 | Figure 2.AdCOMP-Ang1-injected mice show increased synovial proliferation and angiogenesis but no bone destruction. (A) Representative sections of the ankle joints stained with H&E (bars=250 μm), (B) mean pathological scores, and (C) mean radiological scores (n=10 for each group). Note the increased synovial proliferation (asterisk) in the joint of an AdCOMP-Ang1-injected mouse compared with PBS- and AdLacZ-injected mice. (D) Images showing PECAM-1+ blood vessels (bars=50 μm). (E) Density of blood vessels in the synovium (n=5-6 for each group). Note the increased angiogenesis in the synovium of an AdCOMP-Ang1-injected mouse compared with an AdLacZ. Values are the mean± SEM, ∗p<0.05 vs. AdLacZ. |
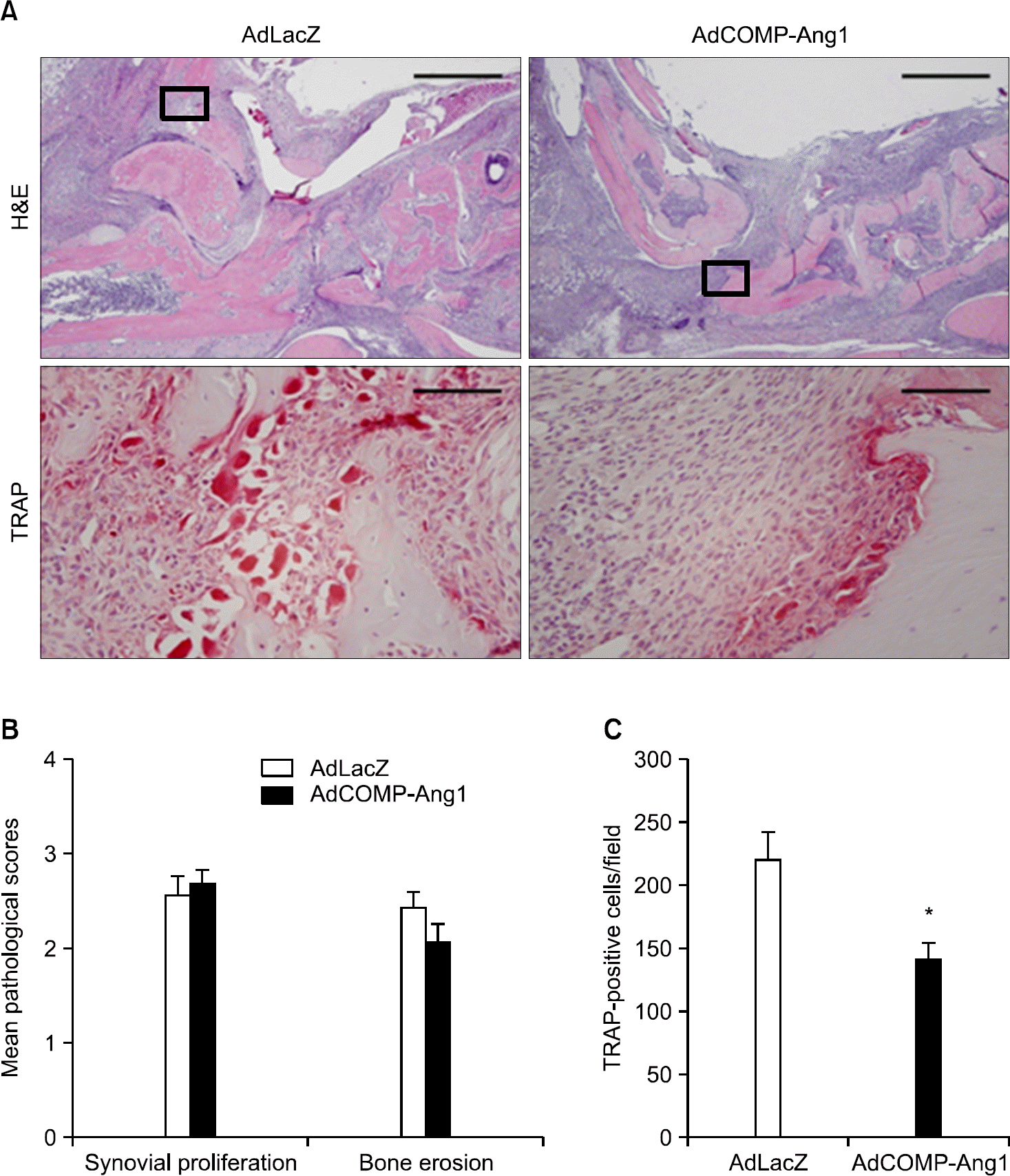 | Figure 3.COMP-Ang1 decreases TRAP-positive osteoclasts. (A) Representative sections of the ankle joints stained with H&E (bars=500 μm) and TRAP (bars=50 μm) from an AdLacZ- or AdCOMP-Ang1-injected mouse with grade 3 synovial proliferation. The TRAP- stained image (lower panel) corres-ponds to the boxed area in the H&E-stained sections (upper panel). (B) Mean pathological scores and (C) total number of TRAP-positive cells in the joints showing grade 3 synovial proliferation from the AdLacZ- or AdCOMP-Ang1-injec-ted groups (n=5 for each group). Values are the mean± SEM, ∗p <0.05. |
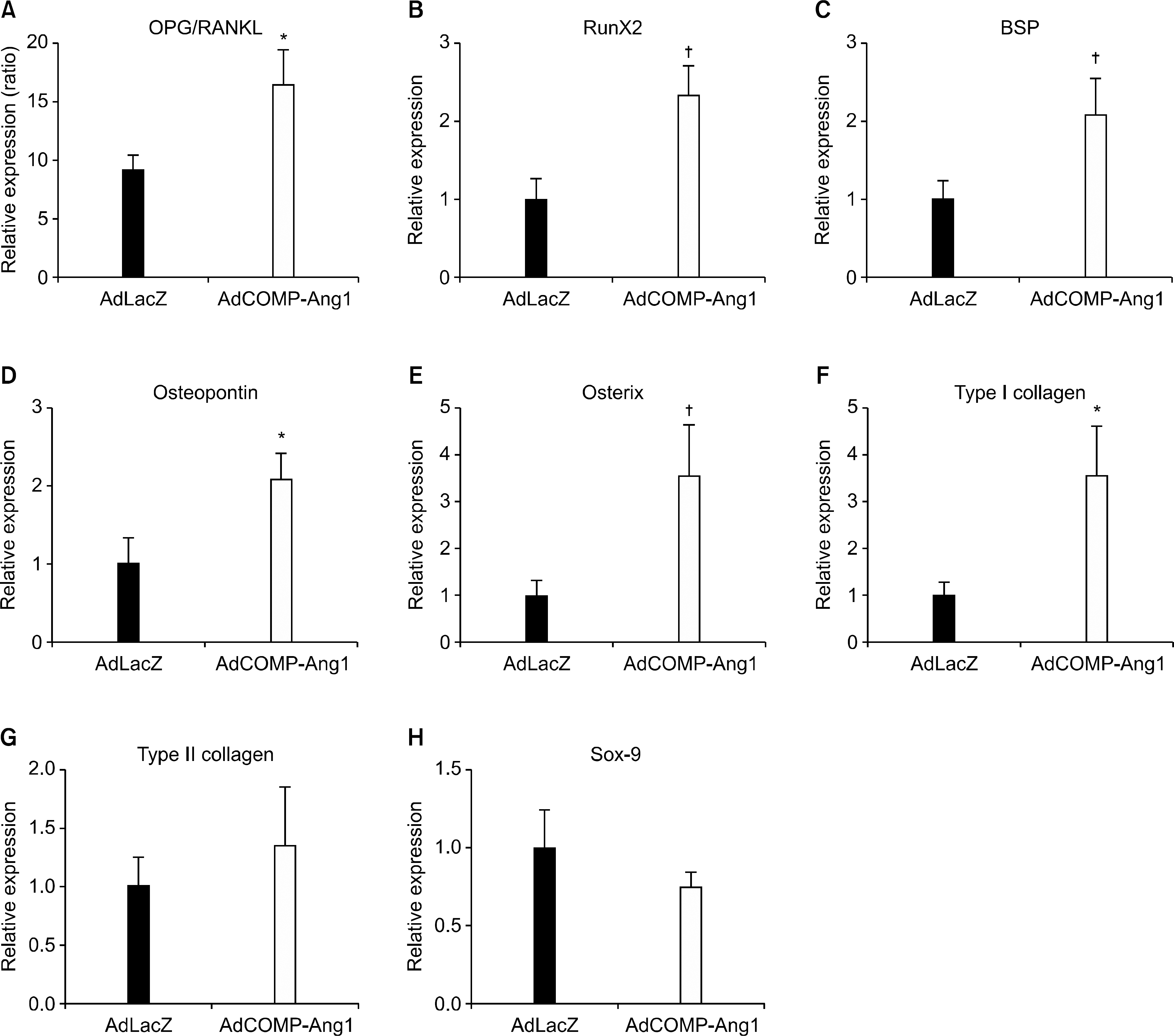 | Figure 4.COMP-Ang1 increases the OPG/RANKL ratio and the expression of the osteogenic genes. Ankle joint tissues were obtained from AdLacZ- and AdCOMP-Ang1-injected mice on day 45 (n=10 for each group). Real-time RT-PCR analysis for osteogenic and chondrogenic genes was performed. Values are the mean± SEM, ∗p<0.05, † p<0.01 vs. AdLacZ. |
Table 1.
Sequences and accession numbers for the forward and reverse primers used in real-time RT-PCR




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download