Abstract
Objective
βig-h3 is a 68kDa extracellular matrix protein which is overexpressed in synovial tissues of rheumatoid arthritis (RA). Previous results proved that βig-h3 fragments are relevant to adhesion and migration of synovial fibroblast and angiogenesis through interaction with αvβ 3 integrin. We designed a recombinant βig-h3 protein consisting of a fas-1 domain and RGD motif and evaluated the therapeutic efficacy in RA.
Methods
Inhibitory effect of adhesion and migration of NIH3T3 cell line was evaluated in 96 well microtiter and transwell plates coated with βig-h3. Clinical arthritis index was evaluated after treating CIA mice with MFK12. Immunohistochemical staining in synovial tissues were performed. Expression of transcripts and proteins of inflammatory mediators were analyzed by semi-quantitative RT-PCR and immunoblotting.
Results
Recombinant protein consisted of 4th fas-1 domain truncated for H1 and H2 sequences and RGD peptide (MFK12), had M.W. of 10.4kDa. βig-h3 mediated adhesion and migration of NIH3T3 cell line were significantly inhibited in a dose-dependent manner. Arthritis severity and incidence were efficiently reduced when CIA mice were treated with MFK12 at 30 mg/kg/day compared with the control. Immunohistochemical staining of joint tissues in MFK12 treated mice exhibited reduced angiogenesis. In treated mice, expression of transcripts regarding inflammatory mediators was markedly suppressed and immunoblotting of ICAM-1 and RANKL from whole extract of hind paws also showed a significant reduction.
Figures and Tables
 | Figure 1Design of the recombinant MFK12 peptide. (A) A schematic representation of pET-29b(+) containing MFK12 sequence. (B) MFK12 peptide was produced by combination of dhfas-1 (amino acid 502-632) with RGD motif. (C) Western blot analysis of purified recombinant MFK12. |
 | Figure 2Effective inhibition of βig-h3-mediated adhesion and migration by MFK12. To elucidate the efficiency of MFK12 in the regulation of cell function, MFK12 was incubated with NIH3T3 cell line in different concentrations before performing adhesion (A) and migration assay (B). Data demonstrates the relative percentage of inhibition by MFK12 compared with that by the control. Results are the mean and SEM of 3 independent experiments (*p<0.05 versus control). |
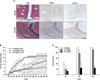 | Figure 3MFK12 induces amelioration of collagen-induced arthritis (CIA) where βig-h3 is unregulated. (A) Immunohistochemical staining of F4/80 and βig-h3 in joint tissues from normal and CIA mice. Expression of F4/80-expressing macrophages and βig-h3 in serial sections of joint tissues (×200). (B&C) Development of arthritis in MFK12-treated mice. From day 22 following the first immunization, mice were treated with MFK12 (0, 10, and 30mg/kg/day, intraperitoneally). (B) Clinical arthritis index was recorded daily. Values are the mean and SEM. (C) Incidence of arthritis among the four paws was recorded at day 30, 40, and 50 after the first immunization. Values are the mean and SEM. *p<0.05 versus the control. |
 | Figure 4Histologies and immunohistologies of joints tissues from control and MFK-12 treated (10 and 30 mg/kg) CIA mice. Expression of CD31 and ICAM-1 was detected in CIA mice. (Hematoxylin counter stained for immunohistochemical stain, ×200). |
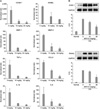 | Figure 5Effect of MFK12 treatment on the expression of inflammatory mediators in CIA mice. (A) Semi-quantitative measurement of transcripts of inflammatory mediators from joint tissues. Transcripts were calculated as target gene/reference gene (18s ribosome RNA) ratios and normalized to the relative expression level of non-arthritis mice. (B) Immunoblotting analysis of ICAM-1 and RANKL for proteins extracted from joint tissues of representative paws. Graphs show the mean and SD optical density (O.D.) from at least 3 independent experiments. The results are means±SD (*p<0.05 versus 0 mg/kg). |
References
1. Knedla A, Neumann E, Müller-Ladner U. Developments in the synovial biology field 2006. Arthritis Res Ther. 2007. 9:209.
2. Firestein GS. Invasive fibroblast-like synoviocytes in rheumatoid arthritis. Passive responders or transformed aggressors? Arthritis Rheum. 1996. 39:1781–1790.
3. Kim JM, Kim KH. Pathogenesis of rheumatoid arthritis. J Korean Med Assoc. 2010. 53:853–861.
4. Barilla ML, Carsons SE. Fibronectin fragments and their role in inflammatory arthritis. Semin Arthritis Rheum. 2000. 29:252–265.
5. Tomasini-Johansson BR, Milbrink J, Pejler G. Vitronectin expression in rheumatoid arthritic synovia--inhibition of plasmin generation by vitronectin produced in vitro. Br J Rheumatol. 1998. 37:620–629.
6. Kim JE, Kim SJ, Lee BH, Park RW, Kim KS, Kim IS. Identification of motifs for cell adhesion within the repeated domains of transforming growth factor-β-induced gene, βig-h3. J Biol Chem. 2000. 275:30907–30915.
7. Skonier J, Bennett K, Rothwell V, Kosowski S, Plowman G, Wallace P, et al. β ig-h3: a transforming growth factor-β-responsive gene encoding a secreted protein that inhibits cell attachment in vitro and suppresses the growth of CHO cells in nude mice. DNA Cell Biol. 1994. 13:571–584.
8. Park SW, Bae JS, Kim KS, Park SH, Lee BH, Choi JY, et al. βig-h3 promotes renal proximal tubular epithelial cell adhesion, migration and proliferation through the interaction with α3β1 integrin. Exp Mol Med. 2004. 36:211–219.
9. Nam JO, Kim JE, Jeong HW, Lee SJ, Lee BH, Choi JY, et al. Identification of the αvβ3 integrin-interacting motif of βig-h3 and its anti-angiogenic effect. J Biol Chem. 2003. 278:25902–25909.
10. Nam JO, Jeong HW, Lee BH, Park RW, Kim IS. Regulation of tumor angiogenesis by fastatin, the fourth FAS1 domain of βig-h3, via αvβ3 integrin. Cancer Res. 2005. 65:4153–4161.
11. Kim JE, Kim SJ, Jeong HW, Lee BH, Choi JY, Park RW, et al. RGD peptides released from βig-h3, a TGF-β-induced cell-adhesive molecule, mediate apoptosis. Oncogene. 2003. 22:2045–2053.
12. Ruoslahti E. RGD and other recognition sequences for integrins. Annu Rev Cell Dev Biol. 1996. 12:697–715.
13. Trabocchi A, Menchi G, Cini N, Bianchini F, Raspanti S, Bottoncetti A, et al. Click-chemistry-derived triazole ligands of arginine-glycine-aspartate (RGD) integrins with a broad capacity to inhibit adhesion of melanoma cells and both in vitro and in vivo angiogenesis. J Med Chem. 2010. 53:7119–7128.
14. Nam EJ, Sa KH, You DW, Cho JH, Seo JS, Han SW, et al. Up-regulated transforming growth factor β-inducible gene h3 in rheumatoid arthritis mediates adhesion and migration of synoviocytes through αvβ3 integrin: Regulation by cytokines. Arthritis Rheum. 2006. 54:2734–2744.
15. Nam EJ, Song EJ, Kim JM, Seo JS, Sa KH, Cho HJ, et al. βig-h3-Mediated Adhesion of Fibroblast-Like Synoviocytes in Rheumatoid Arthritis. J Korean Rheum Assoc. 2008. 15:222–229.
16. Brownlie RJ, Myers LK, Wooley PH, Corrigall VM, Bodman-Smith MD, Panayi GS, et al. Treatment of murine collagen-induced arthritis by the stress protein BiP via interleukin-4-producing regulatory T cells: a novel function for an ancient protein. Arthritis Rheum. 2006. 54:854–863.
17. Kim JE, Jeong HW, Nam JO, Lee BH, Choi JY, Park RW, et al. Identification of motifs in the fasciclin domains of the transforming growth factor-β-induced matrix protein βig-h3 that interact with the αvβ5 integrin. J Biol Chem. 2002. 277:46159–46165.
18. Clemmensen I, Hølund B, Andersen RB. Fibrin and fibronectin in rheumatoid synovial membrane and rheumatoid synovial fluid. Arthritis Rheum. 1983. 26:479–485.
19. Vartio T, Vaheri A, Von Essen R, Isomäki H, Stenman S. Fibronectin in synovial fluid and tissue in rheumatoid arthritis. Eur J Clin Invest. 1981. 11:207–212.
20. Nikkari L, Aho H, Yli-Jama T, Larjava H, Jalkanen M, Heino J. Expression of integrin family of cell adhesion receptors in rheumatoid synovium. α 6 integrin subunit in normal and hyperplastic synovial lining cell layer. Am J Pathol. 1993. 142:1019–1027.
21. Kim JE, Park RW, Choi JY, Bae YC, Kim KS, Joo CK, et al. Molecular properties of wild-type and mutant βIG-H3 proteins. Invest Ophthalmol Vis Sci. 2002. 43:656–661.
22. Sarkissian M, Lafyatis R. Integrin engagement regulates proliferation and collagenase expression of rheumatoid synovial fibroblasts. J Immunol. 1999. 162:1772–1779.
23. LeBaron RG, Bezverkov KI, Zimber MP, Pavelec R, Skonier J, Purchio AF. βIG-H3, a novel secretory protein inducible by transforming growth factor-β, is present in normal skin and promotes the adhesion and spreading of dermal fibroblasts in vitro. J Invest Dermatol. 1995. 104:844–849.
24. Kim JE, Kim EH, Han EH, Park RW, Park IH, Jun SH, et al. A TGF-β-inducible cell adhesion molecule, β ig-h3, is downregulated in melorheostosis and involved in osteogenesis. J Cell Biochem. 2000. 77:169–178.
25. Lee BH, Bae JS, Park RW, Kim JE, Park JY, Kim IS. βig-h3 triggers signaling pathways mediating adhesion and migration of vascular smooth muscle cells through α vβ5 integrin. Exp Mol Med. 2006. 38:153–161.
26. Lutz R, Pataky K, Gadhari N, Marelli M, Brugger J, Chiquet M. Nano-stenciled RGD-gold patterns that inhibit focal contact maturation induce lamellipodia formation in fibroblasts. PLoS One. 2011. 6:e25459.
27. Yoon SH, Mofrad MR. Cell adhesion and detachment on gold surfaces modified with a thiol-functionalized RGD peptide. Biomaterials. 2011. 32:7286–7296.
28. Thomas JP, Arzoomanian RZ, Alberti D, Marnocha R, Lee F, Friedl A, et al. Phase I pharmacokinetic and pharmacodynamic study of recombinant human endostatin in patients with advanced solid tumors. J Clin Oncol. 2003. 21:223–231.
29. Maeshima Y, Yerramalla UL, Dhanabal M, Holthaus KA, Barbashov S, Kharbanda S, et al. Extracellular matrix-derived peptide binds to α(v)β(3) integrin and inhibits angiogenesis. J Biol Chem. 2001. 276:31959–31968.
30. Sudhakar A, Sugimoto H, Yang C, Lively J, Zeisberg M, Kalluri R. Human tumstatin and human endostatin exhibit distinct antiangiogenic activities mediated by αvβ3 and α5β1 integrins. Proc Natl Acad Sci USA. 2003. 100:4766–4771.
31. Hajitou A, Grignet C, Devy L, Berndt S, Blacher S, Deroanne CF, et al. The antitumoral effect of endostatin and angiostatin is associated with a down-regulation of vascular endothelial growth factor expression in tumor cells. FASEB J. 2002. 16:1802–1804.
32. Kato K, Miyake K, Igarashi T, Yoshino S, Shimada T. Human immunodeficiency virus vector-mediated intra-articular expression of angiostatin inhibits progression of collagen-induced arthritis in mice. Rheumatol Int. 2005. 25:522–529.
33. Kim HJ, Kim IS. Transforming growth factor-β-induced gene product, as a novel ligand of integrin αMβ2, promotes monocytes adhesion, migration and chemotaxis. Int J Biochem Cell Biol. 2008. 40:991–1004.
34. Cha DR, Kim IS, Kang YS, Han SY, Han KH, Shin C, et al. Urinary concentration of transforming growth factor-β-inducible gene-h3(βig-h3) in patients with Type 2 diabetes mellitus. Diabet Med. 2005. 22:14–20.
35. Lee SH, Bae JS, Park SH, Lee BH, Park RW, Choi JY, et al. Expression of TGF-β-induced matrix protein β ig-h3 is up-regulated in the diabetic rat kidney and human proximal tubular epithelial cells treated with high glucose. Kidney Int. 2003. 64:1012–1021.
36. Kang YM, Kim SI, Kim JS, You DW, Sa KH, Park EJ, et al. Regulation of βig-h3 production in rheumatoid synovitis by inflammatory madiators. J Korean Rheum Assoc. 2005. 12:73–82.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download