Abstract
The efficacy of anti-TNF-α antibodies including infliximab and adalimumab for refractory intestinal Behcet's disease has recently been demonstrated in a series of case reports. The efficacy of switching to a different kind of anti-TNF-α agent in the face of refractoriness to one kind of anti-TNF-α agent, a common practice of proven efficacy in rheumatoid arthritis, has yet not been reported for intestinal Behcet's disease. In the present study, we report a case of 52-year-old female patient with intestinal Behcet's disease, who lost initial good response to infliximab, and was refractory to subsequent administrations of adalimumab. Her recent relapse of intestinal lesions could be successfully treated with etanercept. This case suggests that switching to etanercept might be a reasonable therapeutic option in case of intestinal Behcet's disease with secondary non-response to anti-TNF-α antibodies that is most likely to be mediated by anti-drug antibody.
Intestinal Behcet's disease is a potentially life-threatening manifestation of Behcet's disease, because refractoriness to conventional treatment such as glucocorticoid, azathioprine and thalidomide can lead to complications including bleeding and perforation resulting in high rates of morbidity and mortality. Recently, there have been a series of case reports on successful use of anti-TNF-α antibodies including infliximab and adalimumab in the induction and maintenance of the remission of refractory intestinal Behcet's disease (1). However, a high rate of recurrence is another difficult aspect of intestinal Behcet's disease, with a reported recurrence rate of 75% in two years in a small group of surgically-treated Behcet's disease patients (2). Therefore, although the exact rates remain obscure, refractoriness to one kind of anti-TNF-α agent may occur throughout the course of treatment. In the case of rheumatoid arthritis, one area in which anti-TNF-α agents are most widely and successfully used for the treatment, switching to a different kind of anti-TNF-α agent is a common and effective way of dealing with refractoriness to an anti-TNF-α agent (3). In this report, we describe a case of intestinal Behcet's disease that showed a loss of initial good response to infliximab and resistence to subsequent adalimumab, in which switching to etanercept resulted in healing of intestinal ulcers.
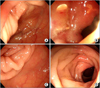
A 52-year-old female patient with known intestinal Behcet's disease presented to a rheumatology outpatient clinic with a complaint of aggravated abdominal pain for the last two weeks. Pain was most severe on the right upper and lower quadrant of the abdomen. There was no history of hematochezia. The patient has recently been on prednisolone 7.5 mg, azathioprine 100 mg once a day. There was mild tenderness on the right upper and lower quadrant of the abdomen. ESR and CRP rose to 52 mm/hr and 2.60 mg/dL, respectively, which were within normal range on the last follow-up three months ago. On colonofiberscopy, there was a huge ulcer on the terminal ileum and ileocecal (IC) valve, with surrounding edema narrowing the lumen of the terminal ileum (Figure 1 A and B). There was mucosal erythema and edema in the ascending colon near the hepatic flexure. The diagnosis of relapse of intestinal Behcet's disease was made.
The patient had been diagnosed with intestinal Behcet's disease at another hospital eighteen years prior to this presentation. The patient reportedly had history of recurrent orogenital ulceration and skin lesions. For the next ten years, the patient had undergone five abdominal surgeries due to persistent disease activity refractory to conventional treatment. Eight years prior to this presentation, infliximab was administered for the first time. Infliximab in combination with prednisolone reportedly lead to a remission of the disease in three months. Months later the patient was lost to follow-up. About two years before this presentation, her disease relapsed. The initial regimen of infliximab in combination with prednisolone did not result in a remission in this time. Subsequently, adalimumab 40 mg biweekly was tried. Six months course of adalimumab in combination with prednisolone did not result in clinically significant change in disease activity. Adalimumab was stopped, and conventional treatments that included glucocorticoid (prednisolone up to 0.5 mg/kg) and azathioprine (1.5~2 mg/kg) were provided in response to the wax and waning courses of the disease. About five months before this presentation, the patient and caregivers requested to retry infliximab remembering its dramatic efficacy in the outset. A two month-course of infliximab given at 0, 2, 6 weeks was not effective. Then the patient visited this hospital.
Considering the lack of alternative choices of proven efficacy, and based on recent reports on the efficacy of etanercept in anti-drug antibody-induced refractoriness to infliximab and adalimumab in rheumatoid arthritis, it was decided to try etanercept. Etanercept 25 mg twice a week was started and the dose of prednisolone was increased to 0.5 mg/kg once a day. One week after the beginning of the treatment, patient reported an improvement of the abdominal pain. By the 7th day CRP dropped to 0.28 mg/dL, and by the 9th day, ESR decreased to 27 mm/hr. Prednisolone was tapered out over the next one month. On colonofiberscopy performed three months after starting etanercept, there was a complete healing of the ulcer on the IC valve, with minimal remnant mucosal edema (Figure 1C and D). Four months after starting etanercept, the patient remains in remission while continuing to take etanercept 25 mg twice a week.
It is generally recommended to initiate treatment of intestinal Behcet's disease with medium to high dose glucocorticoid (prednisolone or its equivalent 0.5~1 mg/kg) in combination with immunosuppressive agents such as azathioprine, sulfasalazine and thalidomide. These opinions are based on a limited number of case reports and retrospective studies. About 40% of patients are expected to respond to the initial treatment, and the recurrence rate at 5 years after complete remission is known to be as high as 50%. Therefore, there has been difficulty in managing patients who experience poor initial response or recurrence.
Since the approval of etanercept by US FDA in 1998, anti-TNF-α agents as a class have improved the treatment of various inflammatory diseases such as Behcet's disease. However, there have been cases of unresponsiveness to an anti-TNF-α agent from outset (primary non-response) and gradual loss of achieved responsiveness (secondary non-response). The formation of neutralizing antibodies against infliximab and adalimumab is one of the reasons of secondary non-response (4). Therefore, in the case of chronic arthritis, two-year drug survival rate of an initial anti-TNF-α agent was 75% when all causes for discontinuation were combined (5). In this instances, switching to another anti-TNF-α agent might be an option, given the scarcity of an alternative agent with proven efficacy. Familiarity of clinicians with the safety profile of anti-TNF-α agents is another reason that switching to other anti-TNF-α agents is frequently considered.
In the case of rheumatoid arthritis, the response to a second anti-TNF-α agent is known to be related to the reason for the failure of the first anti-TNF-α agent. According to the data from British Society of Rheumatology Biologics Register, although the drug survival after switching was favorable with a 6 month-drug survival rate of more than 70%, patients who failed a first agent due to inefficacy were more likely to fail to respond to a second agent due to inefficacy (3). In accordance with this finding, the UK National Institute for Health and Clinical Excellence (NICE) guidance recommends considering switching to another anti-TNF-α agent only when adverse events are the reason for discontinuation (6).
Recently it has was shown that the different causes of initial no response to a anti-TNF-α agent have different implications on the response to subsequent anti-TNF-α treatment. Non-responders to infliximab due to immunogenicity (formation of anti-drug antibodies) had a response rate equivalent to that in TNF-naïve group in response to subsequent treatment with adalimumab or etanercept (7,8). According to these findings, etanercept could be a good alternative agent in face of inefficacy due to immunogenicity, because etanercept is known to be associated with minimal formation of anti-drug antibodies (9). Therefore, although anti-drug antibody could not be measured, switching to etanercept was a rational option in this case because a secondary loss of response due to anti-drug antibodies was the most likely cause of the secondary non-response to infliximab and subsequent adalimumab.
One of interesting aspects of this case is that intestinal manifestation of Behcet's disease responded to etanercept that has not been shown to be effective in Crohn's disease, in contrast to the other anti-TNF-α agents (10). The reasons for the differential efficacy in Crohn's disease between anti-TNF-α antibodies (infliximab and adalimumab) and TNF-α receptor fusion protein (etanercept) remain elusive. One report showed that the difference does not lie in the TNF-α neutralizing capacity but in the capability of anti-TNF-α antibodies, in contrast to etanercept, to bind to and to induce apoptosis in lymphocytes from the peripheral blood and in lamina propria of intestinal lesions. The difference in the binding affinity to lymphocytes was presumably ascribed to limited capacity of etanercept for binding to transmembrane and receptor-bound soluble anti-TNF-α (11).
Although it might not be possible to fully explain the reason for the distinct efficacy of etanercept in intestinal Behcet's disease in this case, there has been evidence that the immuno-pathogenesis of the two diseases is different. In both diseases, Th1 T-cell response is considered to play a dominant role in the pathogenesis (12). However, in intestinal Behcet's disease, heat shock protein 60 and TLR2 expressing cells appear to play important roles in the innate immunity that leads to Th1-skewed adaptive immune response (13). In addition, Th17 response, which has been shown to mediate a number of roles once believed to be mediated by Th1 response in Crohn's disease, does not appear to be as important in intestinal Behcet's disease (14). Therefore, the lack of proven-efficacy of etanercept in Crohn's disease does not preclude its successful use in intestinal Behcet's disease.
In this case, one issue needs to be pointed out. The improvement observed in this case might also be attributable to the co-administered glucocorticoid. However, the dose of prednisolone administered together with etanercept in this case did not exceed the dose of prednisolone the patient took while having persistent symptoms, and a complete remission of intestinal lesions was observed on colonofiberscopy three months later, even though prednisolone was rapidly tapered out over one month after starting etanercept. In addition, there is a report, in which intestinal Behcet's disease complicated by massive gastrointestinal bleeding was successfully treated with infliximab monotherapy (15), providing evidence that in intestinal Behcet's disease, inflammation can be completely controlled by effective TNF-α modulation alone. Therefore, it is unlikely that a significant portion of the clinical improvement observed in this case was originated from prednisolone administered together with etanercept.
Figures and Tables
References
1. Naganuma M, Sakuraba A, Hisamatsu T, Ochiai H, Hasegawa H, Ogata H, et al. Efficacy of infliximab for induction and maintenance of remission in intestinal Behçet's disease. Inflamm Bowel Dis. 2008. 14:1259–1264.
2. Naganuma M, Iwao Y, Inoue N, Hisamatsu T, Imaeda H, Ishii H, et al. Analysis of clinical course and long-term prognosis of surgical and nonsurgical patients with intestinal Behçet's disease. Am J Gastroenterol. 2000. 95:2848–2851.
3. Hyrich KL, Lunt M, Watson KD, Symmons DP, Silman AJ. British Society for Rheumatology Biologics Register. Outcomes after switching from one anti-tumor necrosis factor alpha agent to a second anti-tumor necrosis factor alpha agent in patients with rheumatoid arthritis: results from a large UK national cohort study. Arthritis Rheum. 2007. 56:13–20.
4. Wolbink GJ, Vis M, Lems W, Voskuyl AE, de Groot E, Nurmohamed MT, et al. Development of antiinfliximab antibodies and relationship to clinical response in patients with rheumatoid arthritis. Arthritis Rheum. 2006. 54:711–715.
5. Gomez-Reino JJ, Carmona L. Switching TNF antagonists in patients with chronic arthritis: an observational study of 488 patients over a four-year period. Arthritis Res Ther. 2006. 8:R29.
6. Deighton C, O'Mahony R, Tosh J, Turner C, Rudolf M. Guideline Development Group. Management of rheumatoid arthritis: summary of NICE guidance. BMJ. 2009. 338:b702.
7. Jamnitski A, Bartelds GM, Nurmohamed MT, van Schouwenburg PA, van Schaardenburg D, Stapel SO, et al. The presence or absence of antibodies to infliximab or adalimumab determines the outcome of switching to etanercept. Ann Rheum Dis. 2011. 70:284–288.
8. Bartelds GM, Wijbrandts CA, Nurmohamed MT, Stapel S, Lems WF, Aarden L, et al. Anti-infliximab and anti-adalimumab antibodies in relation to response to adalimumab in infliximab switchers and anti-tumour necrosis factor naive patients: a cohort study. Ann Rheum Dis. 2010. 69:817–821.
9. Dore RK, Mathews S, Schechtman J, Surbeck W, Mandel D, Patel A, et al. The immunogenicity, safety, and efficacy of etanercept liquid administered once weekly in patients with rheumatoid arthritis. Clin Exp Rheumatol. 2007. 25:40–46.
10. Sandborn WJ, Hanauer SB, Katz S, Safdi M, Wolf DG, Baerg RD, et al. Etanercept for active Crohn's disease: a randomized, double-blind, placebo-controlled trial. Gastroenterology. 2001. 121:1088–1094.
11. Van den Brande JM, Braat H, van den Brink GR, Versteeg HH, Bauer CA, Hoedemaeker I, et al. Infliximab but not etanercept induces apoptosis in lamina propria T-lymphocytes from patients with Crohn's disease. Gastroenterology. 2003. 124:1774–1785.
12. Imamura Y, Kurokawa MS, Yoshikawa H, Nara K, Takada E, Masuda C, et al. Involvement of Th1 cells and heat shock protein 60 in the pathogenesis of intestinal Behcet's disease. Clin Exp Immunol. 2005. 139:371–378.
13. Nara K, Kurokawa MS, Chiba S, Yoshikawa H, Tsukikawa S, Matsuda T, et al. Involvement of innate immunity in the pathogenesis of intestinal Behçet's disease. Clin Exp Immunol. 2008. 152:245–251.
14. Ferrante A, Ciccia F, Principato A, Giardina AR, Impastato R, Peralta S, et al. A Th1 but not a Th17 response is present in the gastrointestinal involvement of Behçet's disease. Clin Exp Rheumatol. 2010. 28:4 Suppl 60. S27–S30.
15. Ju JH, Kwok SK, Seo SH, Yoon CH, Kim HY, Park SH. Successful treatment of life-threatening intestinal ulcer in Behçet's disease with infliximab: rapid healing of Behçet's ulcer with infliximab. Clin Rheumatol. 2007. 26:1383–1385.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download