Introduction
Sjögren's syndrome is a chronic autoimmune disease with broad spectrum manifestations, the most prevalent being decreased lacrimal and salivary gland function. It can occur alone (primary Sjögren's syndrome) or in association with other rheumatic diseases (secondary Sjögren's syndrome). Patients with primary Sjögren's syndrome have a wide range of hematologic abnormalities, including hemocytopenia, hypergammaglobulinemia, and monoclonal gammopathy (1). Hemocytopenia is detected in 1/3 of patients, may develop as isolated cytopenia or pancytopenia (2,3). Although these alterations are usually mild without need for treatment, some patients may present severe symptomatic cytopenia (3-5). Thrombocytopenia in Sjögren's syndrome seems to be caused by platelet destruction due to either antiplatelet antibody or immune complex formation (6). Clinically significant severe thrombocytopenia is very rare, only a few cases have been reported in the literature (5,7-9).
We report a rare case of patient with primary Sjögren's syndrome who developed very severe thrombocytopenia.
Case Report
A 50-year-old woman was admitted to the hospital for evaluation of purpura and mucosal bleeding. She had been given a diagnosis of primary Sjögren's syndrome 6 years ago and treated with pilocarpine and tear substitutes. Over this follow-up period, no other rheumatic disease has developed. Two weeks ago, she noticed epistaxis, gum bleeding, and multiple ecchymoses and purpura on her body.
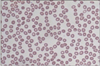
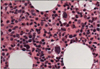
On admission, blood pressure was 120/80 mmHg, temperature 36.8℃, and pulse 75/min. Physical examination revealed widespread purpura and petechiae over trunk and extremities. Heart and lung were normal. Neither organomegaly nor lymphadenopathy was noted. Laboratory tests revealed hemoglobin 10.4 g/dL, white blood cell 7,260/mm3, and platelet 4,000/mm3. Blood chemistry showed protein 8.2 g/dL, albumin 4.1 g/dL, total bilirubin 0.8 mg/dL, AST 25 IU/L, ALT 27 IU/L, creatinine 0.78 mg/dL, LDH 278 IU/L, iron 24 µg/dL, (normal range 70~180) TIBC 294 µg/dL (250~437), and ferritin 22.6 ng/mL (10~290). The prothrombin time and activated partial thromboplastin time were normal. Complement component of C3, C4 and CH50 hemolytic activity were normal range. Antibodies against syphilis, hepatitis B, hepatitis C, or human immunodeficiency virus were not detected. Antinuclear antibody was positive at a titer of 1 : 1,280 with a speckled pattern. Anti-SSA/Ro antibody and rheumatoid factor were positive also. Other antibodies including anti-dsDNA, anti-Smith, anti-SSB/La, anti-Scl70, anti-Jo-1, ANCA (anti-neutrophil cytoplasmic autoantibody), anti-phospholipid, anti-cardiolipin, anti-β2-GP1, lupus anticoagulant, and anti-platelet antibody were all negative. Both direct and indirect Coombs' tests were negative. Urinalysis showed prominent microscopic hematuria. Peripheral blood smear revealed scanty platelets without evidence of hemolysis (Figure 1). Bone marrow examination demonstrated adequate cellularity of megakaryocytes without myelodysplastic or myelofibrotic features (Figure 2). The chest radiography was unremarkable. Ultrasonography of the abdomen revealed no splenomegaly. Schirmer's test was bilaterally positive (1 mm/2 mm), associated with positive Rose Bengal corneal staining. Salivary glands scan disclosed decreased uptake and secretory functions. Therapy with prednisolone (1.0 mg/kg/day) was started. Despite of therapy, the platelet count increase only transiently to 6,000/mm3 and dropped back to 3,000/mm3. Following the failure to respond, she received high dose dexamethasone pulse therapy (40 mg/day for 4 consecutive days) followed by prednisolone 60 mg/day. After this therapy, the purpura and petechiae disappeared and platelet number gradually increased with count of 195,000/mm3. The dose of corticosteroid was slowly tapered with continued stable hematological status. At 12 months after therapy, the platelet count was still normal (208,000/mm3) with prednisolone 2.5 mg/every other day (Figure 3).
Discussion
Patients with primary Sjögren's syndrome often have diverse hematologic manifestations. Ramos-Casals et al. reported the characteristics of the hematologic features in a cross-sectional study. The prevalence of cytopenia was up to 30%. The common cytopenias were anemia (20%), leukopenia (16%), and thrombocytopenia (13%). The degree of thrombocytopenia was mild in most patients, although only 0.4% presented severe thrombocytopenia (platelet count less than 50,000/mm3) (3). Several reports have described the characteristics of thrombocytopenia in patients with Sjögren's syndrome (4,10,11). Most cases of thrombocytopenia were mild and fluctuating over many years. Few cases of symptomatic severe thrombocytopenia have reported (2,5,7-9). One was caused by digitoxin intoxication and treated with corticosteroid and discontinuation of the culprit drug, and the other case was developed as a component of pancytopenia and managed with intravenous immunoglobulin.
Although the cause of thrombocytopenia was not completely defined, it was suggested that peripheral platelet destruction due to either platelet autoantibody or immune-complex reaction (2,6). In the multivariate analysis, cytopenias in Sjögren's syndrome were correlated with several autoantibodies, including antinuclear antibodies (anemia), rheumatoid factor (leukopenia), anti-SSA/Ro (leukopenia), and anti-SSB/La (thrombocytopenia) (3). The causal relation between cytopenia and anti-SSA/Ro or anti-SSB/La antibodies has been suggested. Abnormal cell membrane expression of SSA/Ro or SSB/La antigens might be induced by external agents such as viral infections. A certain viral agent with a specific bone marrow tropism may affect the bone marrow, inducing cell membrane expression of SSA/Ro or SSB/La leading to an autoantibody mediated blood cell destruction (12). Kinds of viruses, such as parvovirus B19 and hepatitis C virus, have been linked to Sjögren's syndrome with cytopenias (6).
The goal of treatment is to raise the platelet count to high enough to prevent bleeding. Conventionally, medium to low dose corticosteroid therapy is the first-line treatment in patients without serious bleeding. In patients experiencing life-threatening bleeding or extremely low platelet counts, high dose corticosteroid pulse therapy is recommended. Cheng et al. reported that dexamethasone pulse therapy was effective for a large number of patients with immune thrombocytopenia (13). Mechanisms of action of corticosteroids on immune thrombocytopenia may be explained as follows: has a direct lymphocytic effect, accelerates lymphocyte apoptosis, inhibits T-cell activation, suppresses B-cell differentiation, and regulates macrophage Fc receptors (13). In patients who are refractory to these treatments, newer therapies including rituximab or the thrombopoetin receptor agonists should be considered.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download