Abstract
Since the 1950's, methotrexatehas been the most widely used for the treatment of rheumatoid arthritis among various disease-modifying anti-rheumatic drugs (DMARDs). In this review, several hidden questions on methotrexate were discussed. First, so far, methotrexate has been considered to improve rheumatoid arthritis by inhibiting cell proliferation through the reduction of synthesis regarding purine and pyrimidine. Recently, a new concept was proposed that methotrexate could increase the release of adenosine, which subsequently decreases the inflammatory function of immune cells, and can finally quench the inflammation in affected joints of rheumatoid arthritis. Second, there were only three clinical trials done to directly compare the efficacy between methotrexate and biologics. With these results, methotrexate showed comparable therapeutic efficacy to biologics, but did not prevent radiological progression. In the future, clinical trials to directly compare the efficacy of methotrexate to biologics will be needed. Third, measuring the serum concentration of methotrexate is not appropriate, since circulating methotrexate is rapidly cleared by cellular uptake or renal excretion. Methotrexate polyglutamate is a more stable compound than methotrexate and it is more likely to relate to efficacy or adverse effects of methotrexate. Recently, the efforts to measure methotrexate polyglutamate in red blood cells have been done to increase therapeutic efficacy and reduce its adverse effects. Fourth, NSAIDs can decrease the excretion of methotrexate though renal tubular cells and it may increase the serum concentration of methotrexate and the risk of its toxicity, suggesting that physicians should pay close attention to dose adjustments concerning methotrexate combined with NSAIDs.
Figures and Tables
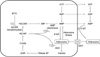
Figure 1
Simplified representation of the effect of methotrexate on adenosine metabolism. Polyglutamate methotrexate inhibits AICAR transformylase, resulting in the intracellular accumulation of AICAR, which inhibits adenosine deaminase and AMP deaminase. Consequently, irreversible degradation of adenosine to inosine is inhibited as well as the conversion of AMP in IMP. Subsequently, AMP is extracellularly converted to adenosine by the ecto-5'-nucleotidase. AICAR, 5-aminoimidazole-4-carboxamide ribonucleotide; ENT, equilibrative nucleoside transporter; FAICAR, 10-formyl AICAR; FGAR, 10-formyl GAR; GAR, glycinamide ribonucleotide; IMP, inosine monophosphate; 5'-NT, 5'-nucleotidase.
References
1. Choy EH, Panayi GS. Cytokine pathways and joint inflammation in rheumatoid arthritis. N Engl J Med. 2001. 344:907–916.
2. Zwerina J, Redlich K, Schett G, Smolen JS. Pathogenesis of rheumatoid arthritis: targeting cytokines. Ann N Y Acad Sci. 2005. 1051:716–729.
3. Paulus HE. The use of combinations of disease-modifying antirheumatic agents in rheumatoid arthritis. Arthritis Rheum. 1990. 33:113–120.
4. Weinblatt ME, Kaplan H, Germain BF, Merriman RC, Solomon SD, Wall B, et al. Methotrexate in rheumatoid arthritis: effects on disease activity in a multicenter prospective study. J Rheumatol. 1991. 18:334–338.
5. van Riel PL, van der Heijde DM, Nuver-Zwart IH, van de Putte LB. Radiographic progression in rheumatoid arthritis: results of 3 comparative trials. J Rheumatol. 1995. 22:1797–1799.
6. Mello SB, Barros DM, Silva AS, Laurindo IM, Novaes GS. Methotrexate as a preferential cyclooxygenase 2 inhibitor in whole blood of patients with rheumatoid arthritis. Rheumatology (Oxford). 2000. 39:533–536.
7. Perkins DJ, St Clair EW, Misukonis MA, Weinberg JB. Reduction of NOS2 overexpression in rheumatoid arthritis patients treated with anti-tumor necrosis factor alpha monoclonal antibody (cA2). Arthritis Rheum. 1998. 41:2205–2210.
8. Scott DL, Kingsley GH. Tumor necrosis factor inhibitors for rheumatoid arthritis. N Engl J Med. 2006. 355:704–712.
9. Silverman GJ, Weisman S. Rituximab therapy and autoimmune disorders: prospects for anti-B cell therapy. Arthritis Rheum. 2003. 48:1484–1492.
10. Sfikakis PP. The first decade of biologic TNF antagonists in clinical practice: lessons learned, unresolved issues and future directions. Curr Dir Autoimmun. 2010. 11:180–210.
11. Bartelds GM, Wijbrandts CA, Nurmohamed MT, Stapel S, Lems WF, Aarden L, et al. Clinical response to adalimumab: relationship to anti-adalimumab antibodies and serum adalimumab concentrations in rheumatoid arthritis. Ann Rheum Dis. 2007. 66:921–926.
12. Radstake TR, Svenson M, Eijsbouts AM, van den Hoogen FH, Enevold C, van Riel PL, et al. Formation of antibodies against infliximab and adalimumab strongly correlates with functional drug levels and clinical responses in rheumatoid arthritis. Ann Rheum Dis. 2009. 68:1739–1745.
13. Gubner R, August S, Ginsberg V. Therapeutic suppression of tissue reactivity. II. Effect of aminopterin in rheumatoid arthritis and psoriasis. Am J Med Sci. 1951. 221:176–182.
14. Phillips DC, Woollard KJ, Griffiths HR. The anti-inflammatory actions of methotrexate are critically dependent upon the production of reactive oxygen species. Br J Pharmacol. 2003. 138:501–511.
15. Nesher G, Osborn TG, Moore TL. In vitro effects of methotrexate on polyamine levels in lymphocytes from rheumatoid arthritis patients. Clin Exp Rheumatol. 1996. 14:395–399.
16. Smith DM, Johnson JA, Turner RA. Biochemical perturbations of BW 91Y (3-deazaadenosine) on human neutrophil chemotactic potential and lipid metabolism. Int J Tissue React. 1991. 13:1–18.
17. Riksen NP, Barrera P, van den Broek PH, van Riel PL, Smits P, Rongen GA. Methotrexate modulates the kinetics of adenosine in humans in vivo. Ann Rheum Dis. 2006. 65:465–470.
18. Montesinos MC, Takedachi M, Thompson LF, Wilder TF, Fernández P, Cronstein BN. The antiinflammatory mechanism of methotrexate depends on extracellular conversion of adenine nucleotides to adenosine by ecto-5'-nucleotidase: findings in a study of ecto-5'-nucleotidase gene-deficient mice. Arthritis Rheum. 2007. 56:1440–1445.
19. Montesinos MC, Desai A, Delano D, Chen JF, Fink JS, Jacobson MA, et al. Adenosine A2A or A3 receptors are required for inhibition of inflammation by methotrexate and its analog MX-68. Arthritis Rheum. 2003. 48:240–247.
20. Nesher G, Mates M, Zevin S. Effect of caffeine consumption on efficacy of methotrexate in rheumatoid arthritis. Arthritis Rheum. 2003. 48:571–572.
21. Benito-Garcia E, Heller JE, Chibnik LB, Maher NE, Matthews HM, Bilics JA, et al. Dietary caffeine intake does not affect methotrexate efficacy in patients with rheumatoid arthritis. J Rheumatol. 2006. 33:1275–1281.
22. Peng Z, Borea PA, Varani K, Wilder T, Yee H, Chiriboga L, et al. Adenosine signaling contributes to ethanol-induced fatty liver in mice. J Clin Invest. 2009. 119:582–594.
23. Peng Z, Fernandez P, Wilder T, Yee H, Chiriboga L, Chan ES, et al. Ecto-5'-nucleotidase (CD73)-mediated extracellular adenosine production plays a critical role inhepatic fibrosis. FASEB J. 2008. 22:2263–2272.
24. Bathon JM, Martin RW, Fleischmann RM, Tesser JR, Schiff MH, Keystone EC, et al. A comparison of etanercept and methotrexate in patients with early rheumatoid arthritis. N Engl J Med. 2000. 343:1586–1593.
25. Breedveld FC, Weisman MH, Kavanaugh AF, Cohen SB, Pavelka K, van Vollenhoven R, et al. The PREMIER study: A multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum. 2006. 54:26–37.
26. Cronstein BN. Low-dose methotrexate: a mainstay in the treatment of rheumatoid arthritis. Pharmacol Rev. 2005. 57:163–172.
27. Danila MI, Hughes LB, Brown EE, Morgan SL, Baggott JE, Arnett DK, et al. Measurement of erythrocyte methotrexate polyglutamate levels: ready for clinical use in rheumatoid arthritis? Curr Rheumatol Rep. 2010. 12:342–347.
28. Stamp LK, O'Donnell JL, Chapman PT, Zhang M, James J, Frampton C, et al. Methotrexate polyglutamate concentrations are not associated with disease control in rheumatoid arthritis patients receiving long-term methotrexate therapy. Arthritis Rheum. 2010. 62:359–368.
29. Stamp LK, O'Donnell JL, Chapman PT, Zhang M, Frampton C, James J, et al. Determinants of red blood cell methotrexate polyglutamate concentrations in rheumatoid arthritis patients receiving long-term methotrexate treatment. Arthritis Rheum. 2009. 60:2248–2256.
30. Dervieux T, Furst D, Lein DO, Capps R, Smith K, Caldwell J, et al. Pharmacogenetic and metabolite measurements are associated with clinical status in patients with rheumatoid arthritis treated with methotrexate: results of a multicentred cross sectional observational study. Ann Rheum Dis. 2005. 64:1180–1185.
31. Hübner G, Sander O, Degner FL, Türck D, Rau R. Lack of pharmacokinetic interaction of meloxicam with methotrexate in patients with rheumatoid arthritis. J Rheumatol. 1997. 24:845–851.
32. Soodvilai S, Chatsudthipong V, Evans KK, Wright SH, Dantzler WH. Acute regulation of OAT3-mediated estrone sulfate transport in isolated rabbit renal proximal tubules. Am J Physiol Renal Physiol. 2004. 287:F1021–F1029.
33. Nozaki Y, Kusuhara H, Kondo T, Iwaki M, Shiroyanagi Y, Nakayama H, et al. Species difference in the inhibitory effect of nonsteroidal anti-inflammatory drugs on the uptake of methotrexate by human kidney slices. J Pharmacol Exp Ther. 2007. 322:1162–1170.
34. Maeda A, Tsuruoka S, Kanai Y, Endou H, Saito K, Miyamoto E, et al. Evaluation of the interaction between nonsteroidal anti-inflammatory drugs and methotrexate using human organic anion transporter 3-transfected cells. Eur J Pharmacol. 2008. 596:166–172.
35. Maeda A, Tsuruoka S, Ushijima K, Kanai Y, Endou H, Saito K, et al. Drug interaction between celecoxib and methotrexate in organic anion transporter 3-transfected renal cells and in rats in vivo. Eur J Pharmacol. 2010. 640:168–171.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download