Abstract
A 58-year-old male patient with chronic hepatitis B infection and hypertension was referred for the evaluation of a skin rash. The skin biopsy showed multiple hyaline thrombi in small blood vessels, red blood cell extravasation, and epidermal atrophy. The CBC, chemistry, UA, and radiological studies were unremarkable except for elevated AST/ALT on liver function tests. The hepatitis B virus markers were compatible with the diagnosis of acute replicative phase chronic hepatitis B; HBeAg 86,646 cpm (count per minute), anti-HBeAb (-), HBV PCR (Quantitative) >1.10×108 IU/mL, and >640,200,000 copies/mL. Rouleaux formation was seen on the peripheral blood smears. Serum PEP/IEP demonstrated an M-spike (27.53%) in the gamma region and abnormal bowed arcs in IgG, kappa light chain with Cryoglobulin (+), ANCA (+), FANA (-), and rheumatoid factor (-) on the serological test. The percent of plasma cells on the bone marrow biopsy was approximately 15%. Type I cryoglobulinemia is a rare disease that can be associated with hematologic disorders, but smoldering myeloma or/and hepatitis B has not been reported in association with Type I cryoglobulinemia. Here, we report a case of Type I cryoglobulinemia that showed multiple skin ulcers due to vascular occlusion related to the monoclonal cryoglobulin with smoldering myeloma and acutely reactivated chronic hepatitis B.
References
1. Brouet JC, Clauvel JP, Danon F, Klein M, Seligmann M. Biologic and clinical significance of cryoglobulins. A report of 86 cases. Am J Med. 1974; 57:775–88.
3. Cohen SJ, Pittelkow MR, Su WP. Cutaneous manifestations of cryoglobulinemia: clinical and histopathologic study of seventy-two patients. J Am Acad Dermatol. 1991; 25:21–7.

4. Rajkumar SV. MGUS and smoldering multiple myeloma: update on pathogenesis, natural history, and management. Hematology Am Soc Hematol Educ Program. 2005; 340–5.

5. Brown LM, Gridley G, Check D, Landgren O. Risk of multiple myeloma and monoclonal gammopathy of un-determined significance among white and black male United States veterans with prior autoimmune, infectious, inflammatory, and allergic disorders. Blood. 2008; 111:3388–94.

7. Agmon-Levin N, Ram M, Barzilai O, Porat-Katz BS, Parikman R, Selmi C, et al. Prevalence of hepatitis C serum antibody in autoimmune diseases. J Autoimmun. 2009; 32:261–6.

8. La Civita L, Zignego AL, Monti M, Longombardo G, Pasero G, Ferri C. Mixed cryoglobulinemia as a possible preneoplastic disorder. Arthritis Rheum. 1995; 38:1859–60.
Figure 1.
Multiple rice sized ery-themas and purpuric papules were seen on the both lower extremities.

Figure 2.
Skin biopsy shows (A) hyaline thrombi in venules (×400, H & E stain), and (B) extravasation of RBC and epidermal atrophy (×200, H & E stain).

Figure 3.
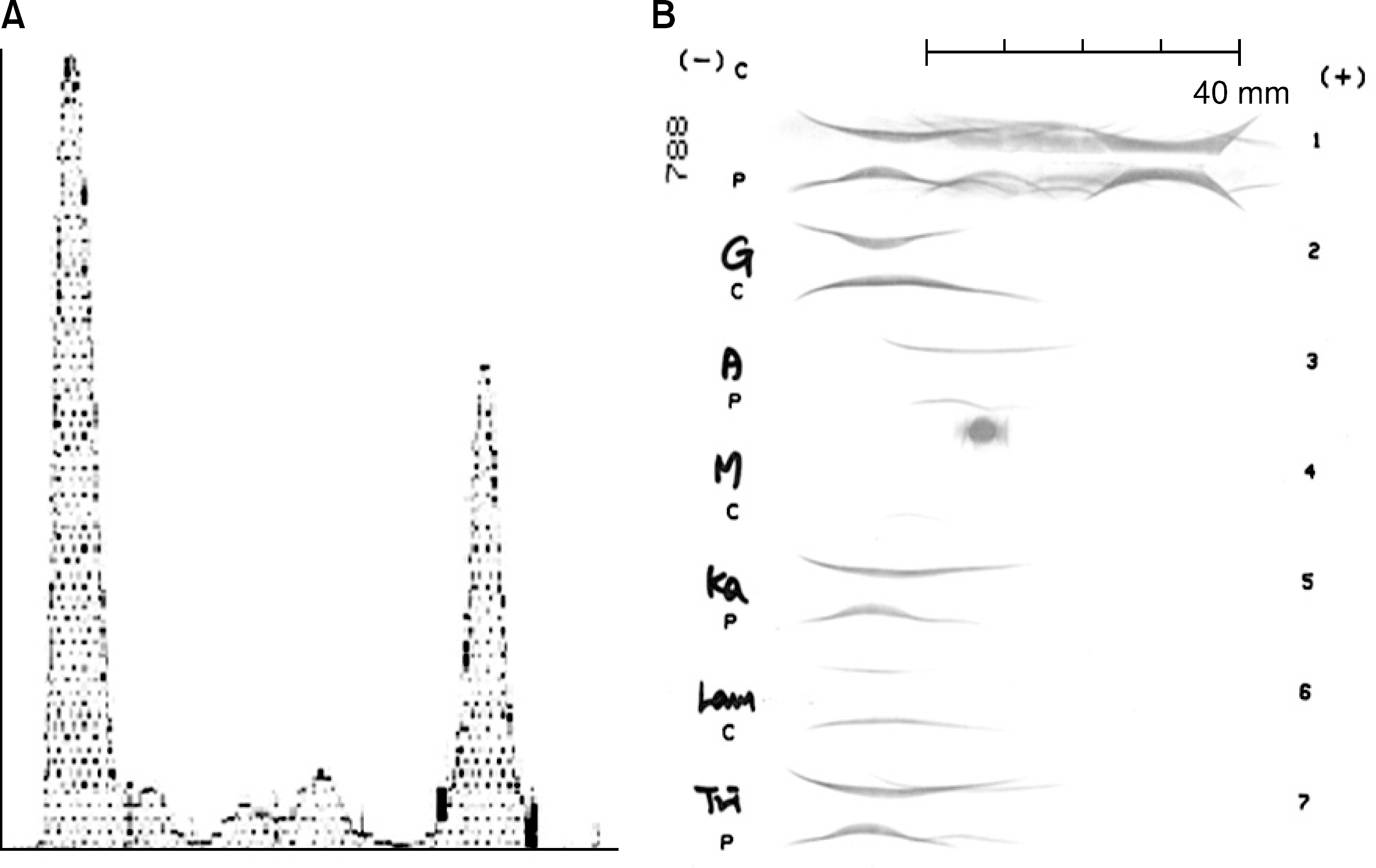
(A) A protein electro-phoresis shows an M-spike. (B) Serum IEP pattern shows moderate increase of gamma globulin area in polyvalent antiserum, and abnormal bowed arcs in IgG, kappa light chain monovalent antisera.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download