Abstract
Objective
We aimed to investigate whether persistence rates of Tumor necrosis factor (TNF) blockers in the early period was affected by the change in reimbursement guideline of Rheumatoid Arthritis (RA) using Korean National Health Insurance (NHI) claims database.
Methods
We identified patients with a diagnosis code of RA between January 2007 to December 2009 and who were 16 years of age or older, in a Korean NHI claims database. A subgroup RA patients who had recently started TNF blockers with 6 months of washout period in June 2007 (n=40), June 2008 (n=60), January 2009 (n=52) and June 2009 (n=68) were selected to compare the 6 months persistence rate. Also, we analyzed a change in prescriptions of TNF blockers in patients with RA for each 6 month period between 2007 and 2009.
Results
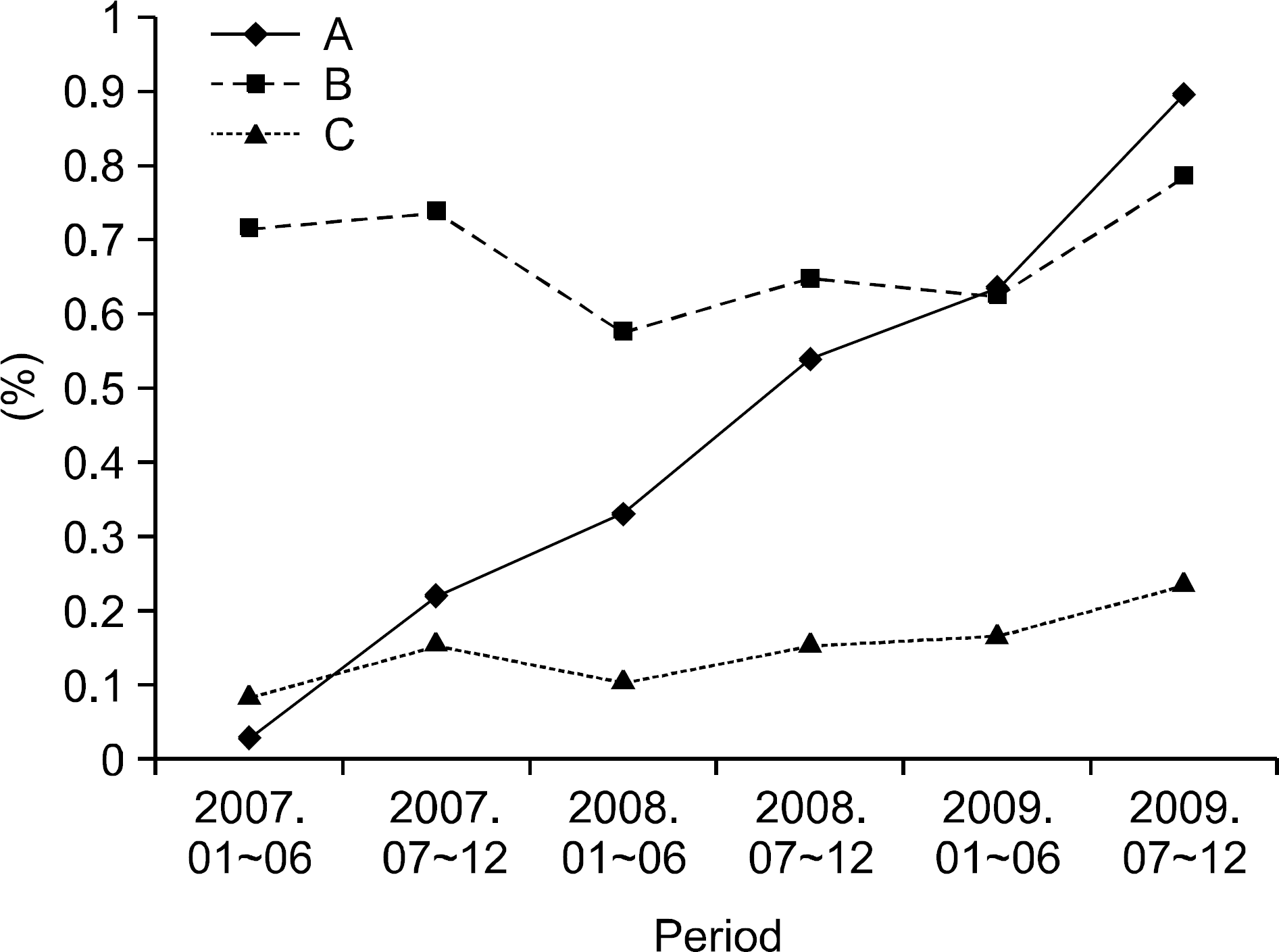
The persistence rates of TNF blockers during 6 months in each group was not statistically significant (67.5%, 75.0%, 73.1%, and 79.4%, p=0.22). However, when we compared the frequency of new patients started on TNF blockers in June 2009 to those in the same months in 2008 and 2007; there was a tendency to increase. During change in TNF blocker prescriptions between 2007 and 2009, the overall utilization of TNF blockers increased.
References
2. Lee SJ, Chang H, Yazici Y, Greenberg JD, Kremer JM, Kavanaugh A. Utilization trends of tumor necrosis factor inhibitors among patients with rheumatoid arthritis in a United States observational cohort study. J Rheumatol. 2009; 36:1611–7.

3. Bressler B, Haraoui B, Keystone E, Sette A. Optimizing use of tumor necrosis factor inhibitors in the management of immune-mediated inflammatory diseases. J Rheumatol Suppl. 2010; 85:40–52.

4. Markenson JA, Gibofsky A, Palmer WR, Keystone EC, Schiff MH, Feng J, et al. Persistence with antitumor necrosis factor therapies in patients with rheumatoid arthritis: observations from the RADIUS registry. J Rheumatol. 2011; 38:1273–81.

5. Choi YS, Lee HY, Kim YH, Paik SJ, Kim JY, Choi YC. Trends and suggestions of improvement in health care utilization on patients with rare and intractable disease. National Health Insurance Coopertaion. 2010.
6. Borah BJ, Huang X, Zarotsky V, Globe D. Trends in RA patients' adherence to subcutaneous anti-TNF therapies and costs. Curr Med Res Opin. 2009; 25:1365–77.

7. Yazici Y, Krasnokutsky S, Barnes JP, Hines PL, Wang J, Rosenblatt L. Changing patterns of tumor necrosis factor inhibitor use in 9074 patients with rheumatoid arthritis. J Rheumatol. 2009; 36:907–13.

8. Bae SC. The current status of surveys on prevalence of rheumatic diseases in Korea. J Korean Rheum Assoc. 2010; 17:1–3.

9. Pascual-Ramos V, Contreras-Yáñez I, Villa AR, Cabiedes J, Rull-Gabayet M. Medication persistence over 2 years of followup in a cohort of early rheumatoid arthritis patients: associated factors and relationship with disease activity and with disability. Arthritis Res Ther. 2009; 11:R26.

10. Konz T, Pentek M, Brodszky V, Ersek K, Orlewska E, Gulacsi L. Adherence to biologic DMARD therapies in rheumatoid arthritis. Expert Opin Biol Ther. 2010; 10:1367–78.
11. Grijalva CG, Chung CP, Arbogast PG, Stein CM, Mitchel EF Jr, Griffin MR. Assessment of adherence to and persistence on disease-modifying antirheumatic drugs (DMA-RDs) in patients with rheumatoid arthritis. Med Care. 2007; 45(10 Supl 2):S66–76.

12. Kristensen LE, Saxne T, Nilsson JA, Geborek P. Impact of concomitant DMARD therapy on adherence to treatment with etanercept and infliximab in rheumatoid arthritis. Results from a six-year observational study in southern Sweden. Arthritis Res Ther. 2006; 8:R174.
13. Curkendall S, Patel V, Gleeson M, Campbell RS, Zagari M, Dubois R. Compliance with biologic therapies for rheumatoid arthritis: do patient out-of-pocket payments matter? Arthritis Rheum. 2008; 59:1519–26.

14. Hyrich KL, Lunt M, Watson KD, Symmons DP, Silman AJ. British Society for Rheumatology Biologics Register. Outcomes after switching from one antitumor necrosis factor alpha agent to a second antitumor necrosis factor alpha agent in patients with rheumatoid arthritis: results from a large UK national cohort study. Arthritis Rheum. 2007; 56:13–20.
15. Du Pan SM, Dehler S, Ciurea A, Ziswiler HR, Gabay C, Finckh A. Swiss Clinical Quality Management Physicians. Comparison of drug retention rates and causes of drug discontinuation between antitumor necrosis factor agents in rheumatoid arthritis. Arthritis Rheum. 2009; 61:560–8.

16. Osiri M, Kamolratanakul P, Maetzel A, Tugwell P. Cost effectiveness analysis of disease modifying antirheumatic drugs in rheumatoid arthritis. Rheumatol Int. 2007; 27:1063–9.

17. DeWitt EM, Glick HA, Albert DA, Joffe MM, Wolfe F. Medicare coverage of tumor necrosis factor alpha inhibitors as an influence on physicians' prescribing beha-vior. Arch Intern Med. 2006; 166:57–63.
18. Yelin E, Callahan LF. The economic cost and social and psychological inpact of musculoskeletal conditions. National Arthritis Data Work Group. Arthritis Rheum. 1995; 38:1351–62.
19. Harley CR, Frytak JR, Tandon N. Treatment compliance and dosage administration among rheumatoid arthritis patients receiving infliximab, etanercept, or methotrexate. Am J Manag Care. 2003; 9(6 Suppl):S136–43.
Table 1.
Baseline characteristics of study population according to change in reimbursement guideline for rheumatoid arthritis
| Characteristics | A (n=40)† | B (n=60)† | C (n=52)† | D (n=68)† | p |
|---|---|---|---|---|---|
| Age, Mean± SD (Min-Max) | 48.73±14.70 (16–82) | 51.30±15.49 (16–75) | 49.31±15.76 (16–72) | 53.37±13.87 (19–82) | 0.3458 |
| Female N (%) | 30 (75.00) | 51 (85.00) | 39 (75.00) | 58 (85.29) | 0.4118 |
| CCI, Mean± SD (Min-Max) | 2.88±1.64 (1–7) | 2.62±1.47 (1–7) | 3.04±1.58 (1–7) | 2.59±1.42 (1–8) | 0.3363 |
| Place of service (N (%)) | |||||
| Tertiary hospital | 31 (77.50) | 39 (65.00) | 37 (71.15) | 48 (70.59) | |
| General hospital | 7 (17.50) | 19 (31.67) | 11 (21.15) | 19 (27.94) | 0.7594 |
| Private clinic | 2 (5.00) | 2 (3.33) | 4 (7.69) | 1 (1.47) | |
| Physician specialty (N (%)) | |||||
| Internal medicine | 35 (87.50) | 57 (95.00) | 49 (94.23) | 67 (98.53) | 0.0292 |
| Other | 5 (12.50) | 3 (5.00) | 3 (5.77) | 1 (1.47) | |
| Persistence rate (N (%))∗ | 27 (67.50) | 45 (75.00) | 38 (73.08) | 54 (79.41) | 0.2222 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download