Abstract
Gout is an inflammatory arthritis characterized by recurrent attacks of inflammation caused by precipitation of monosodium urate crystals (MSU) in the joint and is associated with impaired quality of life. The incidence and prevalence of gout is increasing world wide due to the increasing size of the elderly population, kidney disease, diuretic use, dietary changes, and obesity. Furthermore, emerging evidence suggests that gout is strongly associated with metabolic syndrome and may lead to myocardial in-farction, type 2 diabetes mellitus, and premature death. Urate nephropathy is also increasing. Thus, the overall disease and the economic burden of gout is substantial and increasing sharply. Although traditional urate lowering agents including allopurinol, probenecid, and benzbromar-one have been available for many years, questions still re-main about the optimal dosing regimen. Many patients re-main undertreated for this potentially curable disorder. Fortunately, more scientific data and evidence for proper management of gout are available. Renewed interest in gout has led to advances in dietary advice and development of new therapeutic options. The importance of risk factors for gout should be emphasized and patients with gout and hyperuricemia should be educated so that they can achieve a good quality of life and longevity. In this review, the comorbidity, mortality, and economic burdens of gout will be presented. In addition, the importance of proper management and patient education will be stressed.
Go to : 
References
1. Roddy E, Zhang W, Doherty M. Is gout associated with reduced quality of life? A case-control study. Rheumatology (Oxford). 2007; 46:1441–4.

2. Singh JA, Strand V. Gout is associated with more comorbidities, poorer health-related quality of life and higher healthcare utilisation in US veterans. Ann Rheum Dis. 2008; 67:1310–6.

3. Lee SJ, Hirsch JD, Terkeltaub R, Khanna D, Singh JA, Sarkin A, et al. Perceptions of disease and health-related quality of life among patients with gout. Rheumatology (Oxford). 2009; 48:582–6.

4. Choi HK, Ford ES, Li C, Curhan G. Prevalence of the metabolic syndrome in patients with gout: the Third National Health and Nutrition Examination Survey. Arthritis Rheum. 2007; 57:109–15.

5. Choi HK, Curhan G. Independent impact of gout on mortality and risk for coronary heart disease. Circulation. 2007; 116:894–900.

6. Rho YH, Choi SJ, Lee YH, Ji JD, Choi KM, Baik SH, et al. Prevalence of the metabolic syndrome in patients with gout. J Korean Rheum Assoc. 2004; 11:349–57.
7. Rho YH, Choi SJ, Lee YH, Ji JD, Choi KM, Baik SH, et al. The prevalence of metabolic syndrome in patients with gout: a multicenter study. J Korean Med Sci. 2005; 20:1029–33.

8. Kuo CF, Yu KH, See LC, Chou IJ, Tseng WY, Chang HC, et al. Elevated risk of mortality among gout patients: A comparison with the National Population in Taiwan. Joint Bone Spine. 2011; 78:577–80.

9. Wu EQ, Forsythe A, Guérin A, Yu AP, Latremouille-Viau D, Tsaneva M. Comorbidity burden, healthcare re-source utilization, and costs in chronic gout patients refractory to conventional urate-lowering therapy. Am J Ther In press. 2011.

10. Wallace KL, Riedel AA, Joseph-Ridge N, Wortmann R. Increasing prevalence of gout and hyperuricemia over 10 years among older adults in a managed care population. J Rheumatol. 2004; 31:1582–7.
11. Lawrence RC, Helmick CG, Arnett FC, Deyo RA, Felson DT, Giannini EH, et al. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum. 1998; 41:778–99.

12. Kramer HM, Curhan G. The association between gout and nephrolithiasis: the National Health and Nutrition Examination Survey III, 1988–1994. Am J Kidney Dis. 2002; 40:37–42.

13. Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007–2008. Arthritis Rheum. 2011; 63:3136–41.

14. Currie WJ. Prevalence and incidence of the diagnosis of gout in Great Britain. Ann Rheum Dis. 1979; 38:101–6.

15. Steven MM. Prevalence of chronic arthritis in four geo-graphical areas of the Scottish Highlands. Ann Rheum Dis. 1992; 51:186–94.

16. Harris CM, Lloyd DC, Lewis J. The prevalence and prophylaxis of gout in England. J Clin Epidemiol. 1995; 48:1153–8.

17. Mikuls TR, Farrar JT, Bilker WB, Fernandes S, Schumacher HR Jr, Saag KG. Gout epidemiology: results from the UK General Practice Research Database, 1990–1999. Ann Rheum Dis. 2005; 64:267–72.

18. Annemans L, Spaepen E, Gaskin M, Bonnemaire M, Malier V, Gilbert T, et al. Gout in the UK and Germany: prevalence, comorbidities and management in general practice 2000–2005. Ann Rheum Dis. 2008; 67:960–6.

20. Klemp P, Stansfield SA, Castle B, Robertson MC. Gout is on the increase in New Zealand. Ann Rheum Dis. 1997; 56:22–6.

21. Nan H, Qiao Q, Dong Y, Gao W, Tang B, Qian R, et al. The prevalence of hyperuricemia in a population of the coastal city of Qingdao, China. J Rheumatol. 2006; 33:1346–50.
22. Miao Z, Li C, Chen Y, Zhao S, Wang Y, Wang Z, et al. Dietary and lifestyle changes associated with high prevalence of hyperuricemia and gout in the Shandong coastal cities of Eastern China. J Rheumatol. 2008; 35:1859–64.
23. Kim EH, Jeon K, Park KW, Kim HJ, Ahn JK, Jeon CH, et al. The prevalence of gout among hyperuricemic population. J Korean Rheum Assoc. 2004; 11:7–13.
24. Lee CH, Sung NY. The prevalence and features of Korean gout patients using the national health insurance corporation database. J Rheum Dis. 2011; 18:94–100.

25. Grundy SM, Brewer HB Jr, Cleeman JI, Smith SC Jr, Lenfant C. American Heart Association. National Heart, Lung, and Blood Institute. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004; 109:433–8.
26. Choi HK, Atkinson K, Karlson EW, Curhan G. Obesity, weight change, hypertension, diuretic use, and risk of gout in men: the health professionals followup study. Arch Intern Med. 2005; 165:742–8.
27. Puig JG, Martínez MA, Mora M, Fraile JM, Montoya F, Torres RJ. Serum urate, metabolic syndrome, and cardiovascular risk factors. A population-based study. Nucl-eosides Nucleotides Nucleic Acids. 2008; 27:620–3.

28. Choi HK, De Vera MA, Krishnan E. Gout and the risk of type 2 diabetes among men with a high cardiovascular risk profile. Rheumatology (Oxford). 2008; 47:1567–70.

29. Rathmann W, Funkhouser E, Dyer AR, Roseman JM. Relations of hyperuricemia with the various components of the insulin resistance syndrome in young black and white adults: the CARDIA study. Coronary Artery Risk Development in Young Adults. Ann Epidemiol. 1998; 8:250–61.
30. Mazzali M, Kanellis J, Han L, Feng L, Xia YY, Chen Q, et al. Hyperuricemia induces a primary renal arteriol-opathy in rats by a blood pressure-independent mechanism. Am J Physiol Renal Physiol. 2002; 282:F991–7.
31. Khosla UM, Zharikov S, Finch JL, Nakagawa T, Roncal C, Mu W, et al. Hyperuricemia induces endothelial dysfunction. Kidney Int. 2005; 67:1739–42.

32. Mazzali M, Hughes J, Kim YG, Jefferson JA, Kang DH, Gordon KL, et al. Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension. 2001; 38:1101–6.

33. Sánchez-Lozada LG, Tapia E, Santamaría J, Avila-Casado C, Soto V, Nepomuceno T, et al. Mild hyperuricemia induces vasoconstriction and maintains glomerular hypertension in normal and remnant kidney rats. Kidney Int. 2005; 67:237–47.
34. Johnson RJ, Kang DH, Feig D, Kivlighn S, Kanellis J, Watanabe S, et al. Is there a pathogenetic role for uric acid in hypertension and cardiovascular and renal disease? Hypertension. 2003; 41:1183–90.

35. Grayson PC, Kim SY, LaValley M, Choi HK. Hyperuricemia and incident hypertension: a systematic review and meta-analysis. Arthritis Care Res (Hoboken). 2011; 63:102–10.

37. Rho YH, Woo JH, Choi SJ, Lee YH, Ji JD, Song GG. Association between serum uric acid and the Adult Treatment Panel III-defined metabolic syndrome: results from a single hospital database. Metabolism. 2008; 57:71–6.
38. Nakagawa T, Kang DH, Feig D, Sanchez-Lozada LG, Srinivas TR, Sautin Y, et al. Unearthing uric acid: an an-cient factor with recently found significance in renal and cardiovascular disease. Kidney Int. 2006; 69:1722–5.

39. Kutzing MK, Firestein BL. Altered uric acid levels and disease states. J Pharmacol Exp Ther. 2008; 324:1–7.

40. Tsimberidou AM, Keating MJ. Hyperuricemic syndromes in cancer patients. Contrib Nephrol. 2005; 147:47–60.

43. Kang DH, Nakagawa T, Feng L, Watanabe S, Han L, Mazzali M, et al. A role for uric acid in the progression of renal disease. J Am Soc Nephrol. 2002; 13:2888–97.

44. Kang DH, Yu ES, Park JE, Yoon KI, Kim MG, Kim SJ, et al. Uric acid induced C-reactive protein (CRP) expression via upregulation of angiotensin type 1 receptors (AT1) in vascular endothelial cells and smooth muscle cells. J Am Soc Nephrol. 2003; 14:136A.
45. Kanellis J, Watanabe S, Li JH, Kang DH, Li P, Nakagawa T, et al. Uric acid stimulates monocyte chemoattractant protein-1 production in vascular smooth muscle cells via mitogen-activated protein kinase and cyclooxygenase-2. Hypertension. 2003; 41:1287–93.

46. Kang DH. Does hyperuricemia play a causative role in the development and/or aggravation of renal, cardiovascular and metabolic disease? Korean J Med. 2011; 80:524–8.
47. Siu YP, Leung KT, Tong MK, Kwan TH. Use of allopurinol in slowing the progression of renal disease through its ability to lower serum uric acid level. Am J Kidney Dis. 2006; 47:51–9.

48. Goicoechea M, de Vinuesa SG, Verdalles U, Ruiz-Caro C, Ampuero J, Rincón A, et al. Effect of allopurinol in chronic kidney disease progression and cardiovascular risk. Clin J Am Soc Nephrol. 2010; 5:1388–93.

49. Keenan RT, O'Brien WR, Lee KH, Crittenden DB, Fisher MC, Goldfarb DS, et al. Prevalence of contraindications and prescription of pharmacologic therapies for gout. Am J Med. 2011; 124:155–63.

50. Riedel AA, Nelson M, Joseph-Ridge N, Wallace K, MacDonald P, Becker M. Compliance with allopurinol therapy among managed care enrollees with gout: a retrospective analysis of administrative claims. J Rheumatol. 2004; 31:1575–81.
51. Solomon DH, Avorn J, Levin R, Brookhart MA. Uric acid lowering therapy: prescribing patterns in a large cohort of older adults. Ann Rheum Dis. 2008; 67:609–13.

52. Harrold LR, Andrade SE, Briesacher BA, Raebel MA, Fouayzi H, Yood RA, et al. Adherence with urate-lowering therapies for the treatment of gout. Arthritis Res Ther. 2009; 11:R46.

53. Son KM, Seo YI, Kim IJ, Bae YD, Jung YO, Cha MJ, et al. Adherence to uric acid lowering agent of gouty patients. J Korean Rheum Assoc. 2010; 17:162–7.

54. Edwards NL. Quality of care in patients with gout: why is management suboptimal and what can be done about it? Curr Rheumatol Rep. 2011; 13:154–9.

55. Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006; 440:237–41.

56. Schlesinger N, Mysler E, Lin HY, De Meulemeester M, Rovensky J, Arulmani U, et al. Canakinumab reduces the risk of acute gouty arthritis flares during initiation of allopurinol treatment: results of a double-blind, randomised study. Ann Rheum Dis. 2011; 70:1264–71.

57. Becker MA, Schumacher HR Jr, Wortmann RL, MacDonald PA, Eustace D, Palo WA, et al. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med. 2005; 353:2450–61.

58. Schumacher HR Jr, Becker MA, Wortmann RL, Macdonald PA, Hunt B, Streit J, et al. Effects of febuxostat versus allopurinol and placebo in reducing serum urate in subjects with hyperuricemia and gout: a 28-week, phase III, randomized, double-blind, parallel-group trial. Arthritis Rheum. 2008; 59:1540–8.

59. Beara-Lasic L, Pillinger MH, Goldfarb DS. Advances in the management of gout: critical appraisal of febuxostat in the control of hyperuricemia. Int J Nephrol Renovasc Dis. 2010; 3:1–10.
60. Sundy JS, Becker MA, Baraf HS, Barkhuizen A, Moreland LW, Huang W, et al. Pegloticase Phase 2 Study Investigators. Reduction of plasma urate levels following treatment with multiple doses of pegloticase (polyethylene glycol-conjugated uricase) in patients with treatment-failure gout: results of a phase II randomized study. Arthritis Rheum. 2008; 58:2882–91.

Go to : 
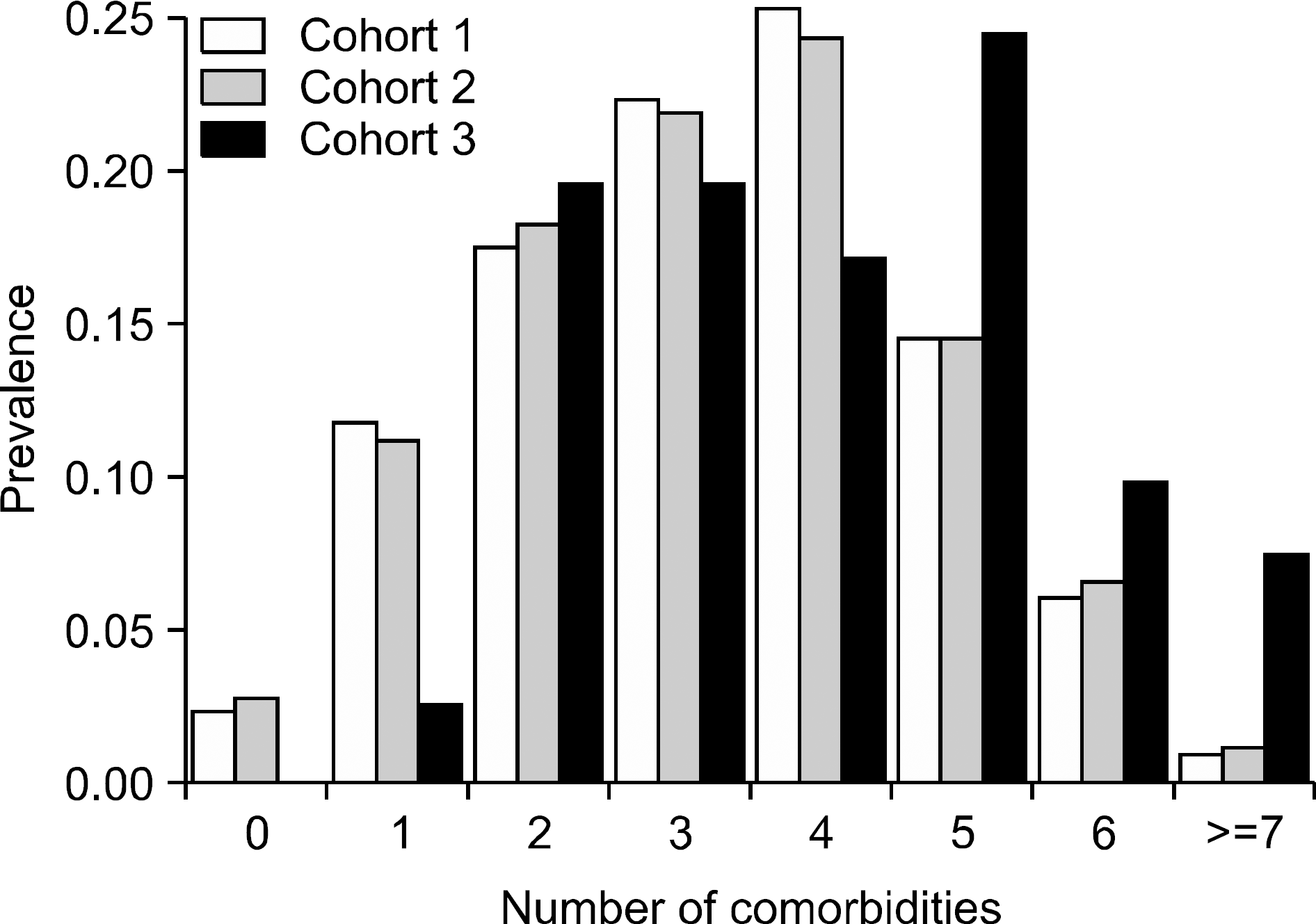 | Figure 1.Patients with gout typically harbor multiple comorbidities. The prevalence of having 0 to 7 associated comorbidities was determined among patients with gout in each of the 3 defined cohorts. |
Table 1.
Unadjusted and age-adjusted comparison of the prevalence of gout and hyperuricemia among US adults between NHANES-III (1988∼1994) and NHANES 2007∼2008∗




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download