Abstract
Objective
To estimate drug persistency and the safety of TNF blocker in Korean patients with rheumatoid arthritis.
Methods
Data were extracted from medical records of rheumatoid arthritis patients who had treated with TNF blocker or are currently using TNF blocker at Hanyang University Hospital for Rheumatic Diseases from Decem-ber 2000 to November 2009 (REtrospective study for Safety and Efficacy of Anti-RA treatment with biologiCs, RESEARCh). Comprehensive chart reviews were under-taken on all patients and data on drug usages and response of TNF blocker was collected at initiation, 3 months and the time of data collection. Persistency with treatment was examined using life-table analysis and multivariate Cox proportional hazard models were developed to examine potential predictors of discontinuation of TNF blocker.
Result
A total of 268 patients were enrolled in this retrospective study. Among them 180 patients were included in the analysis of drug persistency. The 1-year and 5-year drug persistency of TNF blocker was 74% and 46%, respectively. Concomitant use of methotrexate (hazard ratio 0.46, 95% CI 0.27-0.80) was associated with higher persistence. Comparing to etanercept, adalimumab is an independent risk factor for discontinuation (hazard ratio 2.63, 95% CI 1.43-4.84).
Go to : 
References
1. Weinblatt ME, Kremer JM, Bankhurst AD, Bulpitt KJ, Fleischmann RM, Fox RI, et al. A trial of etanercept, a recombinant tumor necrosis factor receptor:Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate. N Engl J Med. 1999; 340:253–9.

2. Breedveld FC, Weisman MH, Kavanaugh AF, Cohen SB, Pavelka K, van Vollenhoven R, et al. The PREMIER study: a multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum. 2006; 54:26–37.

3. Maini R, St Clair EW, Breedveld F, Furst D, Kalden J, Weisman M, et al. Infliximab (chimeric anti-tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. ATTRACT Study Group. Lancet. 1999; 354:1932–9.
4. Navarro-Sarabia F, Ariza-Ariza R, Hernández-Cruz B, Villanueva I. Adalimumab for treating rheumatoid arthritis. J Rheumatol. 2006; 33:1075–81.

5. Zink A, Strangfeld A, Schneider M, Herzer P, Hierse F, Stoyanova-Scholz M, et al. Effectiveness of tumor necrosis factor inhibitors in rheumatoid arthritis in an observational cohort study: comparison of patients according to their eligibility for major randomized clinical trials. Arthritis Rheum. 2006; 54:3399–407.

6. Kievit W, Fransen J, Oerlemans AJ, Kuper HH, van der Laar MA, de Rooij DJ, et al. The efficacy of anti-TNF in rheumatoid arthritis, a comparison between randomised controlled trials and clinical practice. Ann Rheum Dis. 2007; 66:1473–8.

7. Zink A, Askling J, Dixon WG, Klareskog L, Silman AJ, Symmons DP. European biologicals registers: method-ology, selected results and perspectives. Ann Rheum Dis. 2009; 68:1240–6.

8. Watson K, Symmons D, Griffiths I, Silman A. The British Society for Rheumatology biologics register. Ann Rheum Dis. 2005; 64(Suppl 4):iv42–3.
9. Hetland ML. DANBIO: a nationwide registry of bio-logical therapies in Denmark. Clin Exp Rheumatol. 2005; 235(Suppl 39):S205–7.
10. Strangfeld A, Hierse F, Rau R, Burmester GR, Krummel-Lorenz B, Demary W, et al. Risk of incident or recurrent malignancies among patients with rheumatoid arthritis exposed to biologic therapy in the German biologics register RABBIT. Arthritis Res Ther. 2010; 12:R5.

11. Kim YJ, Choi CB, Sung YK, Lee H, Bae SC. Characteristics of Korean patients with RA: a single center cohort Study. J Korean Rheum Assoc. 2009; 16:204–12.

12. Hetland ML. DANBIO–powerful research database and electronic patient record. Rheumatology (Oxford). 2011; 50:69–77.

13. Carmona L, Gómez-Reino JJ. BIOBADASER Group. Survival of TNF antagonists in spondylarthritis is better than in rheumatoid arthritis. Data from the Spanish registry BIOBADASER. Arthritis Res Ther. 2006; 8:R72.
14. Heiberg MS, Koldingsnes W, Mikkelsen K, R⊘devand E, Kaufmann C, Mowinckel P, et al. The comparative one-year performance of antitumor necrosis factor alpha drugs in patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis: results from a longitudinal, observational, multicenter study. Arthritis Rheum. 2008; 59:234–40.
15. Saad AA, Ashcroft DM, Watson KD, Hyrich KL, Noyce PR, Symmons DP. British Society for Rheumatology Biologics Register. Persistence with anti-tumour necrosis factor therapies in patients with psoriatic arthritis: observational study from the British Society of Rheumatology Biologics Register. Arthritis Res Ther. 2009; 11:R52.
16. Duclos M, Gossec L, Ruyssen-Witrand A, Salliot C, Luc M, Guignard S, et al. Retention rates of tumor necrosis factor blockers in daily practice in 770 rheumatic patients. J Rheumatol. 2006; 33:2433–8.
17. Kievit W, Fransen J, Adang EM, den Broeder AA, Bernelot Moens HJ, Visser H, et al. Longterm effectiveness and safety of TNF-blocking agents in daily clinical practice: results from the Dutch Rheumatoid Arthritis Monitoring register. Rheumatology (Oxford). 2011; 50:196–203.

18. Flendrie M, Creemers MC, Welsing PM, den Broeder AA, van Riel PL. Survival during treatment with tumour necrosis factor blocking agents in rheumatoid arthritis. Ann Rheum Dis. 2003; 62(Suppl 2):ii30–3.

19. Kristensen LE, Gülfe A, Saxne T, Geborek P. Efficacy and tolerability of anti-tumour necrosis factor therapy in psoriatic arthritis patients: results from the South Swedish Arthritis Treatment Group register. Ann Rheum Dis. 2008; 67:364–9.

20. Tang B, Rahman M, Waters HC, Callegari P. Treatment persistence with adalimumab, etanercept, or infliximab in combination with methotrexate and the effects on health care costs in patients with rheumatoid arthritis. Clin Ther. 2008; 30:1375–84.

21. Heiberg MS, Koldingsnes W, Mikkelsen K, R⊘devand E, Kaufmann C, Mowinckel P, et al. The comparative one-year performance of antitumor necrosis factor alpha drugs in patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis: results from a longitudinal, observational, multicenter study. Arthritis Rheum. 2008; 59:234–40.
22. Gomez-Reino JJ, Carmona L. BIOBADASER Group. Switching TNF antagonists in patients with chronic arthritis: an observational study of 488 patients over a four-year period. Arthritis Res Ther. 2006; 8:R29.
Go to : 
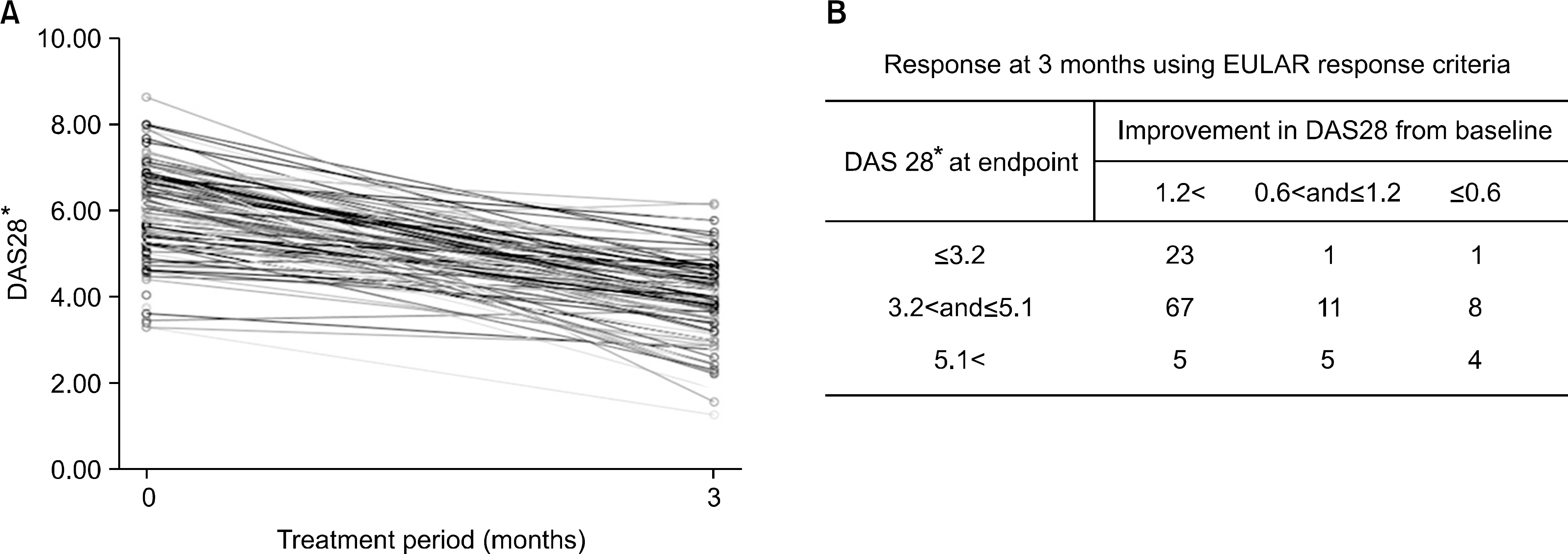 | Figure 2.Response at 3 months. (A) DAS28 change during first 3 months, (B) Response at 3 months using EULAR response criteria, ∗DAS 28 means 3-variable DAS28 using tender joint count, swollen joint count and ESR. |
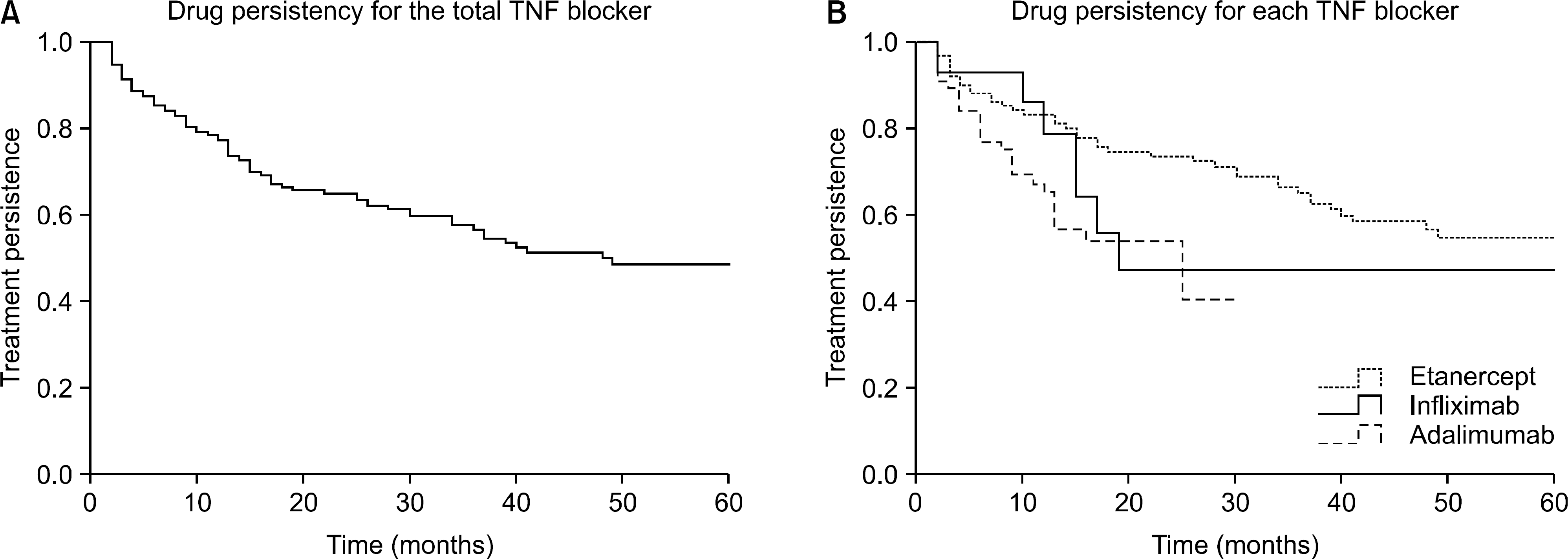 | Figure 3.Drug persistency for TNF blocker in the patients with rheumatoid arthritis. (A) Drug persistency for the total TNF blocker, (B) Drug persistency for each TNF blocker, p=0.007 etanercept vs. adalimumab by Gehan's Wilcoxon method. |
Table 1.
Demographic and clinical features in rheumatoid arthritis patients with TNF blockers
| Subjects for analysis (total n=180) | Number, Mean± SD | Etanercept (n=107) | Adalimumab (n=58) | Infliximab (n=15) | p |
|---|---|---|---|---|---|
| Age at start (years) | 50.5±13.2 | 51.4±12.9 | 48.8±12.9 | 51.3±16.2 | 0.47 |
| Female, No (%) | 155 (86.1) | 92 (86.0) | 51 (87.9) | 12 (80.0) | 0.73 |
| Disease duration at start (years) | 9.5±6.6 | 9.6±6.3 | 9.3±7.0 | 9.5±7.9 | 0.97 |
| RF positive ever, No (%) (n=180) | 130 (72.2) | 83 (77.6) | 36 (62.1) | 11 (73.3) | - |
| Anti-ccp positive, No (%) (n=96) | 83 (86.5) | 36 (85.7) | 44 (88.0) | 3 (75) | - |
| Treatment duration (months) | 26.0±22.3 | 32.2±22.7 | 12.5±8.2 | 33.9±32.9 | <0.001∗ |
Table 2.
Cox proportional hazard analysis for discontinuation
| Variable | HR | 95% CI | p-value | ||
|---|---|---|---|---|---|
| Drug | Infliximab | 1.46 | 0.56∼3.84 | 0.44 | |
| Adalimumab | 2.63 | 1.43∼4.84 | 0.002 | ||
| Etanercept | 1 | ||||
| Gender (Female) | 1.63 | 0.69∼3.82 | 0.26 | ||
| Comorbidity (+) | 0.88 | 0.46∼1.69 | 0.56 | ||
| Disease duration at start∗ | 0.96 | 0.92∼1.01 | 0.08 | ||
| Age at starting TNF blockers | <40 | 1 | |||
| 40∼49 | 1.68 | 0.76∼3.70 | 0.20 | ||
| 50∼59 | 1.06 | 0.49∼2.30 | 0.88 | ||
| 60≤ | 1.28 | 0.58∼2.86 | 0.54 | ||
| Concomitant use of MTX | 0.46 | 0.27∼0.80 | 0.005 | ||
Table 3.
Reasons for discontinuation of TNF blocker users




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download