Abstract
Acromegaly is a clinical syndrome, which is caused by an excess of growth hormone (GH), most commonly secreted from a pituitary solitary adenoma. However, our patient had bilateral GH-secreting pituitary tumors, the incidence of which has been reported in only 1.3 to 1.69% of all acromegalic patients. A 59-year-old female, with no family history of pituitary adenomas, demonstrated an increased level of serum insulin-like growth factor-1 (IGF-1), and GH not suppressed after 75 g oral glucose loading. On a preoperative MRI, only one pituitary tumor, measuring 1.1 × 0.7 cm, could be observed using sellar MRI. After surgical resection of the tumor, her headache and myalgia were sustained, and the IGF-1 level was still in a high titer. Therefore, a follow-up sellar MRI was taken, and a 0.6 × 0.7 cm sized newly growing pituitary tumor was found on the other side. With a retrospective review of radiological examinations, the patient was found to have bilateral tumors. The 0.3 cm sized tumor on the left was too small to be detected on the preoperative MRI. As the patient preferred medical treatment after surgery, she was treated with sandostatin analogues. Acromegaly with bilateral GH-secreting pituitary tumors, is a very rare disease, with no previous case having been reported in Korea. Herein, we report the case with a review of the literature
Figures and Tables
Fig. 1
Two separate pituitary tumors on Sellar Magnetic resonance image (MRI), coronal view. (A) Preoperative sellar MRI shows 1.1 × 0.7 cm sized pituitary adenoma on right sellar wing (arrowhead), and 0.3 cm sized another pituitary adenoma on the left (arrow). (B) Postoperative sellar MRI shows 0.7 × 0.6 cm sized growing state another tumor previously existed (arrow) as shown above.
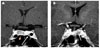
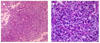
Fig. 2
Histologic findings of the surgical specimen of pituitary adenoma. (A) The fragmented gray white tumor showed solid and cellular lesion with highly vascular structures (H & E stain, × 40). (B) The tumor cells are monotonous and arranged around capillaries forming rossetoid structure (H & E stain, × 400).
References
1. Tolis G, Bertran G, Carpenter S, McKenzie JM. Acromegaly and galactorrhea-amenorrhea with two pituitary adenomas secreting growth hormone or prolactin. Ann Intern Med. 1978. 89:345–348.
2. Kontogeorgos G, Kovacs K, Horvath E, Scheithauer BW. Multiple adenomas of the human pituitary: a retrospective autopsy study with clinical implications. J Neurosurg. 1991. 74:243–247.
3. Kontogeorgos G, Scheithauer BW, Horvath E, Kovacs K, Lloyd RV, Smyth HS, Rologis D. Double adenomas of the pituitary: a clinicopathologic study of 11 tumors. Neurosurgery. 1992. 31:840–849.
4. McKelvie PA, McNeill P. Double pituitary adenomas: a series of three patients. Pathology. 2002. 34:57–60.
5. Meij BP, Lopes MB, Vance MC, Thorner MO, Laws ER Jr. Double pituitary lesions in three patients with Cushing's disease. Pituitary. 2000. 3:159–168.
6. Sano T, Horiguchi H, Xu B, Li C, Hino A, Sakaki M, Kannuki S, Yamada S. Double pituitary adenomas; six surgical cases. Pituitary. 1999. 1:243–250.
7. Blevins LS, Hall GS, Madoff DG, Laws ER, Wand CS. Acromegaly and Cushing's disease in a patient with synchronous pituitary adenomas. Am J Med Sci. 1992. 304:294–297.
8. Kannuki S, Matsumoto K, Sano T, Shintani Y, Baudo H, Saito S. Double pituitary adenoma. Two case reports. Neuro Med Chir. 1996. 36:818–821.
9. Pantelia E, Kontogeorgos G, Piaditis G, Rologis D. Triple pituitary adenomas in Cushing's disease: case report. Acta Neurochir. 1998. 140:190–193.
10. Kovacs K, Ryan N, Horvath E, Singer W, Ezrin C. Pituitary adenomas in old age. J Gerontol. 1980. 35:16–22.
11. McComb DJ, Ryan N, Horvath E, Kovacs K. Subclinical adenomas of the human pituitary. New light on old problems. Arch Pathol Lab Med. 1983. 107:488–491.
12. Ratliff JK, Oldfield EH. Multiple pituitary adenomas in Cushing's disease. J Neurosurg. 2000. 93:753–761.
13. Kim K, Yamada S, Usui M, Sano T. Preoperative identification of clearly separated double pituitary adenomas. Clin Endocrinol. 2004. 61:26–30.
14. Cannavo S, Curto L, Lania A, Saccomanno K, Salpietro FM, Trimarchi F. Unusual MRI finding of multiple adenomas in the pituitary gland: a case report and review of literature. Magn Reson Imaging. 1999. 17:633–636.
16. Freedman RB. Immunosuppresion. Convergence of drug action. Nature. 1989. 341:692.
17. Vallar L, Spada A, Giannattasio G. Altered Gs and adenylate cyclase activity in human GH-secreting pituitary adenomas. Nature. 1987. 330:566–568.
18. Anju S, Rolf J. Bilateral pituitary adenomas occurring with multiple endocrine neoplasia type one. Am J Neuroradiol. 2000. 21:1067–1069.
19. Shintani Y, Yoshimoto K, Horie H, Sano T, Kanesaki Y, Hosoi E, Yokogoshi Y, Bando H, Iwahana H, Kannuki S. Two different pituitary adenomas in a patient with multiple endocrine neoplasia type 1 associated with growth hormone-releasing hormone-producing pancreatic tumor: clinical and genetic features. Endocr J. 1995. 42:331–340.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download