Abstract
Background
Interactions between the receptor activator of the NF-κB ligand (RANKL) and its receptor, RANK, are important in the terminal differentiation and activation of osteoclasts. In the current investigation, we examine the feasibility of using genetically modified mesenchymal stem cells (MSCs), C3H10T1/2 cells as a platform for the sustained systemic delivery of therapeutic proteins into the circulation in an osteoporosis model, and investigate retroviral-mediated gene therapy of RANK-Fc as a means of ameliorating ovariectomy (OVX)-induced bone resorption.
Methods
C3H10T1/2 cells were transduced with a MSCV-based retroviral vector containing cDNA of a fusion protein combining the extracellular domain of murine RANK with the human immunoglobulin constant domain (MSCV-RANK-Fc-eGFP). Young adult female mice were subjected to OVX or sham surgery, followed by treatment with transduced cells or PBS 4 weeks later. The expression of RANK-Fc by these cells was assessed, both in vitro and in vivo. Total bone mineral density (BMD) was measured and GFP expression was examined.
Results
Transduced cells produced biologically active RANK-Fc in vitro and in vivo. Mice that were subjected to OVX followed by treatment with cells transduced with MSCV-RANK-Fc-eGFP 4 weeks later contained no significant but higher total BMD than either the control vector or PBS-treated mice after 8 weeks. Higher GFP expression was attained in the liver, spleen, and intra-abdominal fat of mice treated with MSCV-RANK-Fc-eGFP.
Conclusion
The data collectively indicate that C3H10T1/2 cells are effectively transduced with a MSCV-based retrovirus, and are capable of secreting biologically active RANK-Fc in vitro and in vivo. Moreover, gene therapy facilitating the sustained delivery of RANK-Fc may be an effective method to reverse OVX-induced osteoporosis.
Figures and Tables
Fig. 1
Schematic representation of retroviral vectors. RANK-Fc cDNA is located downstream of MSCV LTR and upstream of IRES-eGFP cassette, allowing the bicistronic expression of both RANK-Fc and eGFP.

Fig. 2
Expression of GFP in transduced C3H10T1/2 cells. The left panels depict light microscopy images, and right panels show images at the same position under fluorescent light. Cells transduced with MSCV-RANK-Fc-eGFP (A) and MSCV-eGFP (B) express GFP (98.3% and 85.3%, respectively) (magnification ×100).

Fig. 3
Cellular expression of RANK-Fc in vitro. Western blot analysis of cellular lysates from transduced Chinese hamster ovarian (CHO) cells. Blots were probed with goat polyclonal anti-mouse RANK antibody (A) and goat polyclonal anti-human IgG Fc fragment-specific antibody (B). Lane 1, pMSCV-eGFP-transduced CHO cells; lane 2, pMSCV-RANK-Fc-eGFP-transduced CHO cells. The numbers on the both sides indicate molecular size markers in kDa.
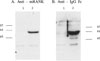
Fig. 4
Western blot analysis of RANK-Fc secreted from C3H10T1/2 cells transduced in vitro. Protein samples from conditioned medium were immunoprecipitated with goat polyclonal anti-mouse RANK antibody and resolved by SDS-PAGE. Blots were probed with goat polyclonal anti-mouse RANK antibody (A) and goat polyclonal anti-human IgG Fc fragment-specific antibody (B). Lane 1, MSCV-RANK-Fc-eGFP-transduced C3H10T1/2 cells; Lane 2, MSCV-eGFP-transduced C3H10T1/2 cells. The numbers on the right signify molecular size markers in kDa.
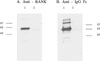
Fig. 5
Systemic physiologic changes observed at 28 days after OVX. Six week-old female C3H mice were sham operated [closed square, n = 4] or ovariectomized [open square, n = 14], and bone mineral density (BMD) (A), bone mineral content (BMC) (B), body weight (C), and body fat mass (D) were measured at baseline and four weeks after the operation. Data are expressed as means and standard deviations.
*P < 0.05, OVX group vs. sham op group (non-parametric Mann-Whitney test).
†P < 0.01, OVX group vs. sham op group (non-parametric Mann-Whitney test).

Fig. 6
RANK-Fc prevents the progression of established osteopenia in C3H mice. A, Schematic representation of the experimental protocol; B, The effect of RANK-Fc on bone mineral density over time. Sham op (closed square, n = 2); OVX + Vehicle (open square, n = 3); OVX +GFP (open circle, n = 2); OVX + RANK-Fc (closed circle, n = 4); C, Changes in bone mineral density after injection; D, Changes in bone mineral density, compared to the baseline. Sham op (open bar, n = 2); OVX + Vehicle (gray bar, n = 3); OVX + GFP (slashed bar, n = 2); OVX + RANK-Fc (black bar, n = 4). Data are expressed as means and standard
deviations.
*P < 0.05, OVX + RANK-Fc vs. OVX + Vehicle (non-parametric Kruskal-Wallis test).
Fig. 7
Whole-body radiographs in C3H mice. C3H mice were injected i.p. with transduced C3H10T1/2 cells or PBS at 4 weeks after OVX or sham op. No significant differences in radiodensity were evident after 8 weeks.
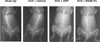
Fig. 8
Production of RANK-Fc in mice. C3H10T1/2 cells were transduced with MSCV-RANK-Fc-eGFP and MSCV-eGFP. Eleven week-old C3H mice were administered transduced cells or PBS on Days 0, 2, 4, and 6 at 4 weeks after OVX. Serum RANK-Fc levels were measured by ELISA on Days 14, 28, and 56 after the first injection. Data are expressed as means and standard deviations (n = 2-4/group).

Fig. 9
Localization of RANK-Fc-secreting cells in tissue sections of transduced C3H10T1/2 cell transplants C3H10T1/2 cells were infected with MSCV-RANK-Fc-eGFP in vitro, and transplanted into ovariectomized C3H mice. After 8 weeks in vivo, transplants were harvested and prepared for the histological evaluation of GFP expression. Higher expression of GFP was attained in liver (A), spleen (B), and intra-abdominal fat (C) in "OVX + RANK-Fc" mice (right), compared with that in corresponding tissues from "sham op" mice (left) (magnification ×100).
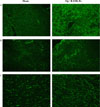
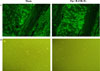
Fig. 10
Localization of RANK-Fc-secreting cells in bone and bone marrow of transplants of transduced C3H10T1/2 cells. C3H10T1/2 cells were infected with MSCV-RANK-Fc-eGFP in vitro, and transplanted into ovariectomized mice. After 8 weeks in vivo, femurs were harvested and prepared for histological evaluation of GFP expression (A), or freshly isolated cell culture from bone marrow was obtained (B) No GFP signal was detected in the "sham op" (left) and "OVX + RANK-Fc" (right) groups (magnification ×100).
References
1. The NIH consensus development panel on osteoporosis prevention, diagnosis, and therapy. JAMA. 2001. 285:785–795.
2. Lopez FJ. New approaches to the treatment of osteoporosis. Curr Opin Chem Biol. 2000. 4:383–393.
3. Fleisch H. Bisphosphonates: mechanisms of action. Endocr Rev. 1998. 19:80–100.
4. Morley P, Whitfield JF, Willick GE. Parathyroid hormone; an anabolic treatment for osteoporosis. Curr Pharm Des. 2001. 7:671–689.
5. Takahashi N, Udagawa N, Suda T. A new member of tumor necrosis factor ligand family, ODF/OPGL/TRANCE/RANKL, regulates osteoclast differentiation and function. Biochem Biophys Res Commun. 1999. 256:449–455.
6. Hofbauer LC, Heufelder AE. Role of receptor activator of nuclear factor-κB ligand and osteoprotegerin in bone cell biology. J Mol Med. 2001. 79:243–253.
7. Simonet WS, Lacey DL, Dunstan CR. Osteoprotegerin: A novel secreted protein involved in the regulation of bone density. Cell. 1997. 89:309–319.
8. Bucay N, Sarosi I, Dunstan CR, Morony S, Tarpley J, Capparelli C, Scully S, Tan HL, Xu W, Lacey DL, Boyle WJ, Simonet WS. Osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev. 1998. 12:1260–1268.
9. Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C, Morony S, Oliveira-dos-Santos AJ, Van G, Itie A, Khoo W, Wakeham A, Dunstan CR, Lacey DL, Mak TW, Boyle WJ, Penninger JM. OPGL is a key regulator ofosteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999. 397:315–323.
10. Li J. RANK is the intrinsic hematopoietic cell surface receptor that controls osteoclastogensis and regulation of bone mass and calcium metabolism. Proc Natl Acad Sci USA. 2000. 97:1566–1571.
11. Hsu H, Lacey DL, Dunstan CR, Solovyev I, Colombero A, Timms E, Tan HL, Elliott G, Kelley MJ, Sarosi I, Wang L, Xia XZ, Elliott R, Chiu L, Black T, Scully S, Capparelli C, Morony S, Shimamoto G, Bass MB, Boyle WJ. Tumor necrosis factor receptor family member RANK mediates osteoclast differentiation and activation induced by osteoprotegerin ligand. Proc Natl Acad Sci USA. 1999. 96:3540–3545.
12. Bolon B, Carter C, Daris M, Morony S, Capparelli C, Hsieh A, Mao M, Kostenuik P, Dunstan CR, Lacey DL, Sheng JZ. Adenoviral delivery of osteoprotegerin ameliorates bone resorption in a mouse ovariectomy model of osteoporosis. Mol Ther. 2001. 3:197–205.
13. Kostenuik PJ, Bolon B, Morony S, Daris M, Geng Z, Carter C, Sheng J. Gene therapy with human recombinant osteoprotegerin reverses established osteopenia inovariectomized mice. Bone. 2004. 34:656–664.
14. Nakashima T, Wada T, Penninger JM. RANKL and RANK as novel therapeutic targets for arthritis. Curr Opin Rheumatol. 2003. 15:280–287.
15. Hofbauer LC, Neubauer A, Heufelder AE. Receptor activator of nuclear factor-κB ligand and osteoprotegerin; potential implications for the pathogenesis and treatment of malignant bone disease. Cancer. 2001. 92:460–470.
16. Zhang J, Dai J, Qi Y, Lin DL, Smith P, Strayhorn C, Mizokami A, Fu Z, Westman J, Keller ET. Osteoprotegerin inhibits prostate cancer-induced osteoclastogenesis and prevents prostate tumor growth in the bone. J Clin Invest. 2001. 107:1235–1244.
17. Body JJ, Greipp P, Coleman RE, Facon T, Geurs F, Fermand JP, Harousseau JL, Lipton A, Mariette X, Williams CD, Nakanishi A, Holloway D, Martin SW, Dunstan CR, Bekker PJ. A phase I study of AMGN-0007, a recombinant osteoprotegerin construct, in patients with multiple myeloma or breast carcinoma related bone metastases. Cancer. 2003. 97:Suppl 3. 887–892.
18. Bekker PJ, Holloway D, Nakanishi A, Arrighi HM, Dunstan CR. The effect of a single dose of osteoprotegerin in postmenopauseal women. J Bone Mineral Res. 2001. 16:348–360.
19. Byrn RA, Mordenti J, Lucas C, Smith D, Marsters SA, Johnson JS, Cossum P, Chamow SM, Wurm FM, Gregory T, Groopman JE, Capon DJ. Biological properties of a CD4 immunoadhesion. Nature. 1990. 344:667–670.
20. Sordillo EM, Pearse RN. RANK-Fc: a therapeutic antagonist for RANK-L in myeloma. Cancer. 2003. 97:Suppl 3. 802–812.
21. Oyajobi BO, Anderson DM, Traianedes K, Williams PJ, Yoneda T, Mundy GR. Therapeutic efficacy of a soluble receptor activator of nuclear factor κB-IgG Fc fusion protein in suppressing bone resorption and hypercalcemia in a model of humoral hypercalcemia of malignancy. Cancer Res. 2001. 61:2572–2578.
22. Pearse RN, Sordillo EM, Yaccoby S, Wong BR, Liau DF, Colman N, Michaeli J, Epstein J, Choi Y. Multiple myeloma disrupts the TRANCE/osteoprotegerin cytokine axis to trigger bone destruction and promote tumor progression. Proc Natl Acad Sci USA. 2001. 98:11581–11586.
23. Emery JG, McDonnell P, Burke MB. Osteoprotegerin is a receptor for the cytotoxic ligand TRAIL. J Biol Chem. 1998. 273:14363–14367.
24. Shipman CM, Croucher PI. Osteoprotegerin is a soluble decoy receptor for tumor necrosis factor-related apoptosis-inducing ligand/Apo2 ligand and can function as a paracrine survival factor for human myeloma cells. Cancer Res. 2003. 63:912–916.
25. Holen I, Croucher PI, Hamdy FC, Eaton CL. Osteoprotegerin (OPG) is a survival factor for human prostate cancer cells. Cancer Res. 2002. 62:1619–1623.
26. Ko KS, Lee MH, Koh JJ, Kim SW. Combined administration of plasmids encoding IL-4 and IL-10 prevents the development of autoimmune diabetes in nonobese diabetic mice. Mol Ther. 2001. 4:313–316.
27. Allay JA, Dennis JE, Haynesworth SE, Majumdar MK, Clapp DW, Shultz LD, Caplan AI, Gerson SL. LacZ and interleukin-3 expression in vivo after retroviral transduction of marrow-derived human osteogenic mesenchymal progenitors. Hum Gene Ther. 1997. 8:1417–1427.
28. Ding LM, Saylors R, Munshi NC. The stromal cell as a vehicle for ex vivo gene transfer. Blood. 1997. 89:446–456.
29. Chuah MKL, Brems H, Vanslembrouck V, Collen D, Vandendriessche T. Bone marrow stromal cells as targets for gene therapy of hemophilia A. Hum Gene Ther. 1998. 9:353–365.
30. Persons DA, Mehaffey MG, Kaleko M, Nienhuis AW, Vanin EF. An improved method for generating retroviral producer clones for vectors lacking a selectable marker gene. Blood Cells Mol Dis. 1998. 24:167–182.
31. Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997. 276:71–74.
32. Prockop DJ, Gregory CA, Spees JL. One strategy for cell and gene therapy: Harnessing the power of adult stem cells to repair tissues. Proc Natl Acad Sci USA. 2003. 100:Suppl 1. 11917–11923.
33. Pereira RF, O'Hara MD, Laptev AV, Halford KW, Pollard MD, Class R, Simon D, Livezey K, Prockop DJ. Marrow stromal cells as a source of progenitor cells for nonhematopoietic tissues in transgenic mice with a phenotype of osteogenesis imperfecta. Proc Natl Acad Sci USA. 1998. 95:1142–1147.
34. Bianco P, Riminucci M, Gronthos S, Robey PG. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells. 2001. 19:180–192.
35. Krebsbach PH, Zhang K, Malik AK, Kurachi K. Bone marrow stromal cells as a genetic platform for systemic delivery of therapeutic proteins in vivo: human factor IX model. J Gene Med. 2003. 5:11–17.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download