Abstract
A 45-year-old woman who complained of weight gain and irregular menstruation was diagnosed as having Cushing's syndrome due to a 3 cm sized left adrenal adenoma. She underwent left adrenalectomy, and she also underwent combined anterior pituitary tests before and 9 months after the surgery. The growth hormone and adrenocorticotropic hormone levels failed to respond to hypoglycemia before the surgery, but their responses recovered after the surgery. Cortisol and thyroid stimulating hormone failed to respond to hypoglycemia and thyrotropin releasing hormone (TRH) before the surgery, respectively, but these were improved after the surgery. Luteinizing hormone, follicle stimulating hormone, and prolactin adequately responded to gonadotropin-releasing hormone and TRH, respectively, before and after the surgery. However, the basal levels of these hormones were higher after adrenalectomy, suggesting that hypercortisolemia had a significant influence on all the pituitary hormones.
Figures and Tables
Fig. 1
Abdominal CT scan shows round mass on leftadrenal gland measured by approximately 3 cm in diameter.
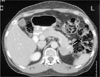
Fig. 2
Histopathologic findings. The adrenal cortical adenoma is well defined and partly capsulated and surrounded by compressed medullar (arrow) and atrophic cortical gland (H&E stain, ×40). The tumor is composed of variable cells showing vacuolated bright cells and eosinophilic dark cells (inset, H&E stain, ×400).

Fig. 3
Insulin tolerance test. Time-course response of adrenocorticotropic hormone (ACTH) (A), cortisol (B), and growth hormone (GH) (C) concentrations to insulin injection was evaluated before and after the adrenal surgery.

Fig. 4
Thyrotropin releasing hormone (TRH) loading test. Time-course responses of thyroid stimulating hormone (TSH) (A) and prolactin (B) to TRH (200 µg IV) were evaluated before and after the adrenal surgery.

Fig. 5
Gonadotropin releasing hormone (GnRH) loading test. Time-course response of follicle stimulating hormone (FSH) (A) and luteinizing hormone (LH) (B) to GnRH (100 µg IV) injection was evaluated before and after the adrenal surgery.

References
1. Hartog M, Gaafar MA, Fraser R. Effect of corticosteroids on serum growth hormone. Lancet. 1964. 2:376–378.
2. Demura R, Demura H, Nunokawa T, Baba H, Miura K. Responses of plasma ACTH, GH, LH, and 11-hydroxycorticosteroids to various stimuli in patients with Cushing's syndrome. J Clin Endocrinol Metab. 1972. 34:852 –859.
3. Hashimoto K. The pituitary ACTH, GH, LH, FSH, TSH and prolactin reserves in patients with Cushing's syndrome. Endocrinol Jpn. 1975. 22:67–77.
4. Cushing WH. The basophilic adenomas of the pituitary body and theirclinical manifestations (pituitary basophilism). Bull John Hopkins Hosp. 1932. 50:137–195.
6. Orth DN. Cushing's syndrome. N Eng J Med. 1995. 332:791–803.
7. Watanabe K, Adachi A, Nakamura R. Reversible panhypopituitarism due to Cushing's syndrome. Arch Intern Med. 1988. 148:1358–1360.
8. Cuedra C, Estrada J, Marazuela M, Vicente A, Astigarraga B, Bernabeu I, Diez S, Salto L. Anterior pituitary function in Cushing's syndrome: study of 36 patients. Endocrinol Jpn. 1991. 38:559–563.
9. Senaris RM, Lago F, Coya R, Pineda J, Dieguez C. Regulation of hypothalamic somatostatin, growth hormone-releasing hormone, and growth hormone receptor messenger ribonucleic acid by glucocorticoids. Endocrinology. 1996. 137:5236–5241.
10. Wehrenberg WB, Bergman PJ, Staag L, Ndon J, Guistina A. Glucocorticoid inhibition of growth hormone in rats: partial reversal with somatostatin antibodies. Endocrinology. 1990. 127:2705–2708.
11. Leal-Cerro A, Soto A, Martinez MA, Alvarez P, Isidro L, Casanueva F, Dieguez C, Cordido F. Effect of withdrawal of somatostatin plus growth hormone (GH)-releasing hormone as a stimulus of GH secretion in Cushing's syndrome. Clin Endocrinol (Oxf). 2002. 57:745–749.
12. Ozata M, Dieguez C, Casanueva F. The inhibition of growth hormone secretion presented in obesity is not mediated by the high leptin levels: a study in human leptin deficiency patients. J Clin Endocrinol Metab. 2003. 88:312–316.
13. Haigler ED Jr, Pittman JA Jr, Hershman JM, Baugh CM. Direct evaluation of pituitary thyrotropin reserve utilizing synthetic thyrotropin release hormone. J Clin Endocrinol Metab. 1971. 33:573–581.
14. Otsuki M, Dakoda M, Baba S. Influence of glucocorticoids on TRF-induced TSH response in man. J Clin Endocrinol Metab. 1973. 36:95–102.
15. Sowers JR, Carlson HE, Brautbar N, Hershman JM. Effects of dexamethasone on prolactin and TSH responses to TRH and metoclopromide in man. J Clin Endocrinol Metab. 1977. 44:237–241.
16. Hubina E, Nagy GM, Toth BE, Ivan G, Gorombey Z, Szaboles I, Kovacs L, Goth MI. Dexamethasone and adrenocorticotropin suppress prolactin secretion in humans. Endocrine. 2002. 18:215–219.
17. Kasperlik-Zaluska AA, Jeske W. Serum prolactin responses to metoclopromide in Cushing's syndrome and Nelson's syndrome. . Acta Endocrinol (Copenh). 1980. 93:351–355.
18. Stolp R, Bevers MM, Rijnberk A, Croughs RJ, Rutterman GR. Regulation of prolactin secretion in canine pituitary-dependent hyperadrenocorticism. Horm Metab Res. 1986. 18:595–598.
19. Inagaki K, Otsuka F, Miyoshi T, Watanabe N, Suzuki J, Ogura R, Makino H. Reversible pituitary dysfunction in a patient with Cushing's syndrome discovered as adrenal incidentaloma. Endocr J. 2004. 51:201–206.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download