Abstract
Methimazole is a widely used and generally well-tolerated antithyroid agent. Adverse reactions occur in
1~5% of patients taking methimazole medication, but these are most commonly transient, benign leukopenia
and a skin rash. Severe cholestatic jaundice, combined with agranulocytosis, has been known as a rare
complication. Herein, a case of methimazole induced cholestatic jaundice, with agranulocytosis, is reported.
Figures and Tables
Fig. 1
Abdominal Computed tomography showed unremarkable liver, gallbladder, pancreas, and spleen, without evidence of biliary dilatation.
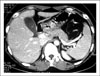
Fig. 2
Time course of white cell counts and body temperature in relation to treatment (G-CSF: Granulocyte-Colony Stimulating Factor, WBC: White Blood Cell, ANC:Absolute Granulocyte Count).

References
1. Marisa CW, Joao HR, Nazareth B, Rubens SW, Chady SF. Adverse Effects Related to Thionamide Drugs and their dose regimen. Am J Med Sci. 1989. 297:216–219.
3. Cooper DS, Goldminz D, Levin AA. Agranulocytosis associated with antithyroid drugs. Effects of patient age and drug dose. Ann Inern Med. 1983. 98:26–29.
5. Mikhail NE. Methimazole-induced cholestatic jaundice. South Med J. 2004. 97:178–182.
6. Specht NW, Boehme EJ. Death due to agranulocytosis induced by methimazole therapy. JAMA. 1952. 149:1010–1011.
7. Rosenbaum H, Reveno WS. Agranulocytosis and toxic hepatitis from methimazole. JAMA. 1953. 152:27.
8. Shipp J. Jaundice during methimazole(Tapazole) administration. Ann Intern Med. 1955. 42:701–706.
9. Manojlovic D, Nesovic M, Micic J, Duric D. Agranulocytose ot hepatite chronique chez les toalades traites par favistan. Srpskl Archiv Za Celokupno Lekarstro. 1977. 105:549–554.
10. International agranulocytosis and aplastic anemia study group. Risk of agranulocytosis and aplastic anemia in relation to use of antithyroid drugs. Br Med J. 1988. 297:262–265.
11. Douer D, Eisenstein Z. Methimazole-induced agranulocytosis: growth inhibition of myeloid progenitor cells by the patient's serum. Eur J Haematol. 1988. 40:91–94.
12. Palmblad J, Johnson B, Kanerud L. Treatment of drug-induced agranulocytosis with recombinant granulocyte, macropahge-colony stimulating factor. J Intern Med. 1990. 228:537–542.
13. Heinrich B, Gross M, Gobel FD. Methimazole- Induced Agranulocytosis and Granulocyte-Colony Stimulating Factor. Ann of Int Med. 1989. 111:621–622.
14. Fukata S, Kuma K, Sugawara M. Granulocyte colony-stimulating factor(G-CSF) dose not improve recovery from antithyroid drug-induced agranulocytosis: a prospective study. Thyroid. 1999. 9:29–31.
15. Vitug AC, Goldman JM. Hepatotoxicity from antithyroid drugs. Hrom Res. 1985. 21:229–234.
16. Becker CE, Gorden P, Robbins J. Hepatitis from methimazole during adrenal steroid therapy for malignant exophthalmos. JAMA. 1968. 206:1787–1789.
17. AntaonAranda E. Intrahepatic cholestasis in untreated hyperthyroidism. Rev Esp Enferm Dig. 2000. 92:49–50.
18. Kravetz D. Intrahepatic cholestasis caused by danantizol. Rev Esp Enferm Apar Dig. 1972. 38:725–738.
19. Fisher MG, Nayer HR, Miller A. Methimazole-induced jaundice. JAMA. 1973. 223:1028–1029.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download