Abstract
Background
The gonadotropin releasing hormone (GnRH) neurons represent the final output cells of the neural network that controls fertility. Dopamine (DA) has been shown to control gonadotropin release in many species. However, the direct membrane effects of DA and the related receptors on GnRH neurons remain poorly understood. The purpose of this study was to investigate the direct actions of DA on GnRH neurons and the related receptors using brain slice electrophysiology.
Methods
Gramicidin-perforated patch clamp recordings were made from the GnRH neurons to examine the direct membrane effects of DA in GnRH-EGFP mut5 mice.
Results
DA induced hyperpolarization of the GnRH neurons, which was maintained in the presence of tetrodotoxin (TTX), a Na+ channel blocker, suggesting a direct, rather than indirect, action of DA on GnRH neurons. DA-induced hyperpolarizing effects were blocked by prazosin, an α1-adrenergic antagonist, and mimicked by phenylephrine (PE), an α1-adrenergic agonist.
Figures and Tables
Fig. 1
This trace shows the process of making pores. Usually, 5 to 20 minutes after gigaseal formation, gramicidin starts to make pores. When gramicidin starts to make pores, resting membrane potential (RMP) is decreased by making pores. When the RMP was stable, DA and other chemicals tested were applied. In perforated mode, access resistance between the pipette and cell was approximately 40 to 100 MΩ. The arrow indicates the point when the perforated mode becomes whole cell mode, and then the access resistance is lowered below 10 MΩ and shows overshooting of action potentials.

Fig. 2
Gramicidin perfroated-patch recordings showing the hyperpolarizing responses of GnRH neurons to dopamine (DA). A, GnRH neurons showed a hyperpolarizing response to 1 min exposure to 100 μM DA (74 day-old, RMP=-59 mV). B, DA-induced hyperpolarizing response was persisted in the presence of tetrodotoxin (TTX, 0.5 μM, 74 day-old, RMP=-59 mV).
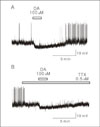
Fig. 3
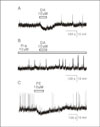
Hyperpolarizing effect of dopamine (DA) on GnRH neurons was mediated by α1 adrenergic receptor. A. DA (10 μM) hyperpolarized a GnRH neuron (48 day-old, RMP = -60 mV). B. DA-induced hyperpolarizing response was blocked by prazosin (Pra, 10 μM), an α1-adrenergic antagonist (70 day-old, RMP = -66 mV). C. DA-induced hyperpolarizing response was mimicked by phenylephrine (PE, 10 μM), an α1-adrenergic agonist (48 day-old, RMP = -60 mV).
References
1. Herbison AE. Henry H, Norman A, editors. GnRH neuron. Encyclopedia of hormones. 2003. Vol 2. San Diego: Academic Press;171–177.
2. Clarke IJ, Cummins JT. The temporal relationship between gonadotropin releasing hormone (GnRH) and luteinizing hormone (LH) secretion in ovariectomized ewes. Endocrinology. 1982. 111:1737–1739.
3. Deaver DR, Dailey RA. Effects of dopamine and serotonin on concentrations of luteinizing hormone and estradiol-17β in plasma of cycling ewes. Biol Reprod. 1983. 28:870–877.
4. Meyer SL, Goodman RL. Neurotransmitters involved in mediating the steroid-dependent suppression of pulsatile luteinizing hormone secretion in anestrous ewes: effects of receptor antagonists. Endocrinology. 1985. 116:2054–2061.
5. Curlewis JD, Naylor AM, McNeilly AS. Evaluation of a possible role for the dopamine D1 and D2 receptors in the steroid-dependent suppression of luteinizing hormone secretion in the seasonally anoestrous ewe. J Neuroendocrinol. 1991. 3:387–391.
6. Tortonese DJ, Lincoln GA. Photoperiodic modulation of the dopaminergic control of pulsatile LH secretion in sheep. J Endocrinol. 1994. 143:25–32.
7. Chang JP, Peter RE. Effects of dopamine on gonadotropin release in female goldfish, Carassius auratus. Neuroendocrinology. 1983. 36:351–357.
8. Chang JP, Peter RE, Nahorniak CS, Sokolowska M. Effects of catecholaminergic agonists and antagonists on serum gonadotropin concentration and ovulation in goldfish: evidence for specificity of dopamine inhibition of gonadotropin secretion. Gen Comp Endocrinol. 1984. 55:351–360.
9. Omeljaniuk RJ, Shih SH, Peter RE. In-vivo evaluation of dopamine receptor-mediated inhibition of gonadotropin secretion from the pituitary gland of the goldfish, Carrassius auratus. J Endocrinol. 1987. 114:449–458.
10. Yu KL, Peter RE. Adrenergic and dopaminergic regulation of gonadotropin releasing hormone release from goldfish preoptic anterior hypothalamus and pituitary in vitro. Gen Comp Endocrinol. 1992. 85:138–146.
11. Goodman RL. Functional organization of the catecholaminergic neural systems inhibiting luteinizing hormone secretion in anestrous ewes. Neuroendocrinology. 1989. 50:406–412.
12. Weiner RI, Martinez de la Escalera G. Pulsatile release of gonadotropin releasing hormone (GnRH) is an intrinsic property of GT1 GnRH neuronal cell lines. Hum Reprod. 1993. 8:Suppl 2. 13–17.
13. Han SK, Todman MG, Herbison AE. Endogenous GABA release inhibits the firing of adult gonadotropin releasing hormone neurons. Endocrinology. 2004. 145:495–499.
14. Ebihara S, Shirato K, Harata N, Akaike N. Gramicidin-perforated patch recording: GABA response in mammalian neurons with intact intracellular chloride. J Physiol. 1995. 484:77–86.
15. Silverman AJ, Witkin JW. Biocynthesis of gonadotropin-releasing hormone during the rat estrous cycle: a cellular analysis. Neuroendocrinology. 1994. 59:545–551.
16. Herbison AE. Noradrenergic regulation of cyclic Gn-RH secretion. Rev Reprod. 1997. 2:1–6.
17. Xia L, Van Vugt D, Alston EJ, Luckhaus J, Ferin M. A surge of gonadotropin-releasing hormone accompanies the estradiol-inuduced gonadotropin surge in the rhesus monkey. Endocrinology. 1992. 131:2812–2820.
18. Karsch FJ, Bowen JM, Caraty A, Evans NP, Moenter SM. Gonadotropin-releasing hormone requirements for ovulation. Biol Reprod. 1997. 56:303–309.
19. Watanabe M, Sakuna Y, Kato M. High expression of the R-type voltage gated Ca2+ channel and its involvement in Ca2+ dependent gonadotropin releasing hormone release in GT1-7 cells. Endocrinology. 2004. 145:2375–2383.
20. Costantin JL, Charles AC. Modulation of Ca2+ signaling by K+ channels in a hypothalamic neuronal cell line (GT1-1). J Neurophysiol. 2001. 85:295–304.
21. Wetsel WC, Eraly SA, Whyte DB, Mellon PL. Regulation of gonadotropin releasing hormone by protein kinase A and C in immortalized hypothalamic neurons. Endocrinology. 1993. 132:2360–2370.
22. Rhee JS, Ebihara S, Akaike N. Gramicidin perforated patch-clamp technique reveals glycine-gated outward chloride current in dissociated nucleus solitari neurons of the rat. J Neurophysiol. 1994. 72:1103–1108.
23. Contijoch AM, Gonzalez C, Singh HN, Malamed S, Troncoso S, Advis JP. Dopaminergic regulation of luteinizing hormone-releasing hormone release at the median eminence level: immunocytochemical and physiological evidence in hens. Neuroendocrinology. 1992. 55:290–300.
24. Leranth C, MacLusky NJ, Shanabrough M, Naftolin F. Catecholaminergic innervation of luteinizing hormone-releasing hormone and lutamic acid decarboxy lase immunopositive neurons in the rat medial preoptic area An electron-microscopic double immunostaining and degeneration study. Neuroendocrinology. 1984. 48:591–602.
25. Cornil CA, Balthazart J, Motte P, Massotte L, Seutin V. Dopamine activates noradrenergic receptors in the preoptic area. J Neurosci. 2002. 22:9320–9330.
26. Ooi H, Colucci WS. Hardman JG, Limbird LE, Gilman AG, editors. Pharmacological treatment of heart failure. The pharmacological basis of therapeutics. 2001. 10th ed. New York: McGraw-Hill;901.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download