Abstract
Background
Growing evidence has shown a biochemical link between increased oxidative stress and reduced bone density. In our previous study, α-lipoic acid (α-LA), a thiol antioxidant, suppressed both osteoclastogenesis and bone resorption, and also prevented TNF-α-induced apoptosis of osteoblast lineages. The effects of α-LA were investigated on bone metabolism in rats with a low bone mass.
Methods
An ovariectomy (OVX) or Talc injection (inflammation-mediated osteopenia, IMO) was performed in 12 week old female Sprague-Dawley rats. Diets containing either 0.3%, 0.5% or 1.0% α-LA were administered to the OVX rats for 16 weeks, and to the IMO rats for 21 days. The bone mineral densities (BMD) of the anterior-posterior lumbar spine and total femur were measured using dual-energy X-ray absorptiometry (Hologic QDR 4500-A), with small animal software. The plasma bone specific alkaline phosphatase activity (BSAP) and urinary free deoxypyridinoline concentration (DPD) were determined using enzyme immunoassay methods.
Results
The body weights were significantly decreased in the OVX rats on the diets containing 0.3 and 0.5% α-LA than in the OVX control. No significant differences in the BMD at either site were noted between rats administered the diets with or without α-LA. However, the administration of various doses of α-LA noticeably decreased the level of urinary DPD in both the OVX and IMO rats. High doses of α-LA (0.5% and/or 1.0%) also decreased the levels of plasma BSAP in both models.
Figures and Tables
Fig. 1
Experimental protocols in ovariectomized rats (A) and inflammation-mediated osteopenia model (B).

Fig. 2
Change in body weights during the experiment with ovariectomized rats. Study groups consist of sham operated rats with standard diet (I), ovariectomized rats with standard diet (II), ovariectomized rats with diet containing 0.3% α-lipoic acid (α-LA) (IV), and ovariectomized rats with diet containing 0.5% α-LA (V). The OVX pair-fed control (III) was ovariectomized rats given the same amount of standard diet as that consumed by 0.5% α-LA group. *P < 0.05, **P < 0.001 vs. sham OP control.

Fig. 3
Bone mineral density (BMD) at the lumbar spine (A) and total femur (B) during the experiment with ovariectomized rats. Study groups consist of sham operated rats with standard diet (I), ovariectomized rats with standard diet (II), ovariectomized rats with diet containing 0.3% α-lipoic acid (α-LA) (IV), and ovariectomized rats with diet containing 0.5% α-LA (V). The OVX pair-fed control (III) was ovariectomized rats given the same amount of standard diet as that consumed by 0.5% α-LA group.
*P < 0.05, **P < 0.01 vs. sham OP control.

Fig. 4
Body weights (A), and bone mineral density (BMD) at the lumbar spine (B) and total femur (C) during the experiment with inflammation-mediated osteopenia models (IMO). Study groups consist of saline injected rats with standard diet (I), IMO rats with standard diet (II), IMO rats with diet containing 0.3% (III), 0.5% (IV) and 1.0% α-lipoic acid (α-LA) (V). *P < 0.05, vs. saline control.
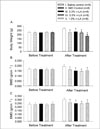
Table 1
Plasma Bone Specific Alkaline Phosphatase (BSAP) and Urinary Deoxypyridinoline/Creatinine Ratio (DPD) at 16 weeks of Study day in Sham Controls, Ovariectomized (OVX) Controls, OVX Pair-Fed Controls, and OVX rats Treated with Diets Containing 0.3% and 0.5% α-lipoic acid (α-LA)

References
1. McCord JM. The evolution of free radicals and oxidative stress. Am J Med. 2000. 108:652–659.
2. Sontakke AN, Tare RS. A duality in the roles of reactive oxygen species with respect to bone metabolism. Clin Chim Acta. 2002. 318:145–148.
3. Basu S, Michaelsson K, Olofsson H, Johansson S, Melhus H. Association between oxidative stress and bone mineral density. Biochem Biophys Res Commun. 2001. 288:275–279.
4. Maggio D, Barabani M, Pierandrei M, Polidori MC, Catani M, Mecocci P, Senin U, Pacifici R, Cherubini A. Marked decrease in plasma antioxidants in aged osteoporotic women: results of a cross-sectional study. J Clin Endocrinol Metab. 2003. 88:1523–1527.
5. Weber P. The role of vitamins in the prevention of osteoporosis-a brief status report. Int J Vitam Nutr Res. 1999. 69:194–197.
6. Hall SL, Greendale GA. The relation of dietary vitamin C intake to bone mineral density: results from the PEPI study. Calcif Tissue Int. 1998. 63:183–189.
7. Patrick L. Toxic metals and antioxidants: Part II. The role of antioxidants in arsenic and cadmium toxicity. Altern Med Rev. 2003. 8:106–128.
8. Melhus H, Michaelsson K, Holmberg L, Wolk A, Ljunghall S. Smoking, antioxidant vitamins, and the risk of hip fracture. J Bone Miner Res. 1999. 14:129–135.
9. Silverton SF, Mesaros S, Markham GD, Malinski T. Osteoclast radical interactions: NADPH causes pulsatile release of NO and stimulates superoxide production. Endocrinology. 1995. 136:5244–5247.
10. Yang S, Ries WL, Key LL Jr. Nicotinamide adenine dinucleotide phosphate oxidase in the formation of superoxide in osteoclasts. Calcif Tissue Int. 1998. 63:346–350.
11. Garrett IR, Boyce BF, Oreffo RO, Bonewald L, Poser J, Mundy GR. Oxygen-derived free radicals stimulate osteoclastic bone resorption in rodent bone in vitro and in vivo. J Clin Invest. 1990. 85:632–639.
12. Damoulis PD, Hauschka PV. Nitric oxide acts in conjunction with proinflammatory cytokines to promote cell death in osteoblasts. J Bone Miner Res. 1997. 12:412–422.
13. Hukkanen M, Corbett SA, Batten J, Konttinen YT, McCarthy ID, Maclouf J, Santavirta S, Hughes SP, Polak JM. Aseptic loosening of total hip replacement. Macrophage expression of inducible nitric oxide synthase and cyclo-oxygenase-2, together with peroxynitrite formation, as a possible mechanism for early prosthesis failure. J Bone Joint Surg Br. 1997. 79:467–474.
14. Mogi M, Kinpara K, Kondo A, Togari A. Involvement of nitric oxide and biopterin in proinflammatory cytokine-induced apoptotic cell death in mouse osteoblastic cell line MC3T3-E1. Biochem Pharmacol. 1999. 58:649–654.
15. Chen RM, Liu HC, Lin YL, Jean WC, Chen JS, Wang JH. Nitric oxide induces osteoblast apoptosis through the de novo synthesis of Bax protein. J Orthop Res. 2002. 20:295–302.
16. Parhami F, Jackson SM, Tintut Y, Le V, Balucan JP, Territo M, Demer LL. Atherogenic diet and minimally oxidized low density lipoprotein inhibit osteogenic and promote adipogenic differentiation of marrow stromal cells. J Bone Miner Res. 1999. 14:2067–2078.
17. Mody N, Parhami F, Sarafian TA, Demer LL. Oxidative stress modulates osteoblastic differentiation of vascular and bone cells. Free Radic Biol Med. 2001. 31:509–519.
18. Packer L, Witt EH, Tritschler HJ. Alpha-Lipoic acid as a biological antioxidant. Free Radic Biol Med. 1995. 19:227–250.
19. Packer L. Alpha-Lipoic acid: a metabolic antioxidant which regulates NF-kappa B signal transduction and protects against oxidative injury. Drug Metab Rev. 1998. 30:245–275.
20. Roy S, Sen CK, Tritschler HJ, Packer L. Modulation of cellular reducing equivalent homeostasis by alpha-lipoic acid. Mechanisms and implications for diabetes and ischemic injury. Biochem Pharmacol. 1997. 53:393–399.
21. Jacob S, Streeper RS, Fogt DL, Hokama JY, Tritschler HJ, Dietze GJ, Henriksen EJ. The antioxidant alpha-lipoic acid enhances insulin-stimulated glucose metabolism in insulin-resistant rat skeletal muscle. Diabetes. 1996. 45:1024–1029.
22. Henriksen EJ, Jacob S, Streeper RS, Fogt DL, Hokama JY, Tritschler HJ. Stimulation by alpha-lipoic acid of glucose transport activity in skeletal muscle of lean and obese Zucker rats. Life Sci. 1997. 61:805–812.
23. Ziegler D, Reljanovic M, Mehnert H, Gries FA. Alpha-lipoic acid in the treatment of diabetic polyneuropathy in Germany: current evidence from clinical trials. Exp Clin Endocrinol Diabetes. 1999. 107:421–430.
24. Packer L, Tritschler HJ, Wessel K. Neuroprotection by the metabolic antioxidant alpha-lipoic acid. Free Radic Biol Med. 1997. 22:359–378.
25. Koh JM, Lee YS, Byun CH, Chang EJ, Kim H, Kim YH, Kim HH, Kim GS. Alpha-lipoic acid suppresses osteoclastogenesis despite increasing the receptor activator of nuclear factor kappaB ligand/osteoprotegerin ratio in human bone marrow stromal cells. J Endocrinol. 2005. 185:401–413.
26. Byun CH, Koh JM, Kim DK, Park SI, Lee KU, Kim GS. Alpha-lipoic acid inhibits TNF-alpha-induced apoptosis in human bone marrow stromal cells. J Bone Miner Res. 2005. 20:1125–1135.
27. Minne HW, Pfeilschifter J, Scharla S, Mutschelknauss S, Schwarz A, Krempien B, Ziegler R. Inflammation-mediated osteopenia in the rat: a new animal model for pathological loss of bone mass. Endocrinology. 1984. 115:50–54.
28. Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J, Boyle WJ. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998. 93:165–176.
29. Iotsova V, Caamano J, Loy J, Yang Y, Lewin A, Bravo R. Osteopetrosis in mice lacking NF-κB1 and NF-κB2. Nat Med. 1997. 3:1285–1289.
30. Bertolini DR, Nedwin GE, Bringman TS, Smith DD, Mundy GR. Stimulation of bone resorption and inhibition of bone formation in vitro by human tumour necrosis factors. Nature. 1986. 319:516–518.
31. Biller BM, Saxe V, Herzog DB, Rosenthal DI, Holzman S, Klibanski A. Mechanisms of osteoporosis in adult and adolescent women with anorexia nervosa. J Clin Endocrinol Metab. 1989. 68:548–554.
32. Lucas AR, Melton LJ III, Crowson CS, O'Fallen WM. Long-term fracture risk among women with anorexia nervosa: a population-based cohort study. Mayo Clin Proc. 1999. 74:972–977.
33. Kalu DN, Masoro EJ, Yu BP, Hardin RR, Hollis BW. Modulation of age-related hyperparathyroidism and senile bone loss in Fischer rats by soy protein and food restriction. Endocrinology. 1988. 122:1847–1854.
34. Klibanski A, Biller BM, Schoenfeld DA, Herzog DB, Saxe VC. The effects of estrogen administration on trabecular bone loss in young women with anorexia nervosa. J Clin Endocrinol Metab. 1995. 80:898–904.
35. Ducy P, Amling M, Takeda S, Priemel M, Schilling AF, Beil FT, Shen J, Vinson C, Rueger JM, Karsenty G. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000. 100:197–207.
36. Takeda S, Elefteriou F, Levasseur R, Liu X, Zhao L, Parker KL, Armstrong D, Ducy P, Karsenty G. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002. 111:305–317.
37. Elefteriou F, Ahn JD, Takeda S, Starbuck M, Yang X, Liu X, Kondo H, Richards WG, Bannon TW, Noda M, Clement K, Vaisse C, Karsenty G. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature. 2005. 434:514–520.
38. Kim MS, Park JY, Namkoong C, Jang PG, Ryu JW, Song HS, Yun JY, Namgoong IS, Ha J, Park IS, Lee IK, Viollet B, Youn JH, Lee HK, Lee KU. Anti-obesity effects of alpha-lipoic acid mediated by suppression of hypothalamic AMP-activated protein kinase. Nat Med. 2004. 10:727–733.
39. Viollet B, Andreelli F, Jorgensen SB, Perrin C, Ge loen A, Flamez D, Mu J, Lenzner C, Baud O, Bennoun M, Gomas E, Nicolas G, Wojtaszewski JF, Kahn A, Carling D, Schuit FC, Birnbaum MJ, Richter EA, Burcelin R, Vaulont S. The AMP-activated protein kinase alpha2 catalytic subunit controls wholebody insulin sensitivity. J Clin Invest. 2003. 111:91–98.
40. Lean JM, Davies JT, Fuller K, Jagger CJ, Kirstein B, Partington GA, Urry ZL, Chambers TJ. A crucial role for thiol antioxidants in estrogen-deficiency bone loss. J Clin Invest. 2003. 112:915–923.
41. Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996. 273:59–63.
42. Carr MC. The emergence of the metabolic syndrome with menopause. J Clin Endocrinol Metab. 2003. 88:2404–2411.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download