Abstract
Acromegaly is a systemic endocrine disorder due to an excessive release of growth hormone, which increases the serum levels of insulin-like growth factor-1 (IGF-1). Elevated levels of these hormones are assumed to increase the incidence of malignant tumors in patients with acromegaly, due to by stimulating the growth and maturation of cells. In particular, IGF-1 is considered to be closely related with the development of colon polyps and colon cancers. Studies suggest that various malignant tumors, including thyroid cancer, brain tumor and renal cell carcinomas, are also more common in patients with acromegaly. Here, a case of gall bladder cancer in a patient with acromegaly, and the possible relationships between these two disorders, is reported.
Figures and Tables
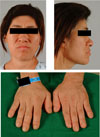 | Fig. 1Acral bony overgrowth results in increased hand and foot size, and prognathism; soft tissue swelling results in coarse facial features and large fleshy nose. |
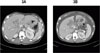
 | Fig. 2Sella MRI: There is a 3.2×2.5 cm sized, heterogeneously enhancing, irregular mass lesion. It extends into suprasella lesion and invasively extends into right side carvenous sinus, while encircling right carotid artery |
References
1. Colao A, Ferone D, Marzullo P, Lombardi G. Systemic complications of acromegaly: epidemiology, pathogenesis, and management. Endocr Rev. 2004. 25:102–152.
2. Baris D, Gridley G, Ron E, Weiderpass E, Mellemkjaer L, Ekbom A, Olsen JH, Baron JA, Fraumeni JF Jr. Acromegaly and cancer risk, A cohort study in Sweden and Denmark. Cancer Cause Control. 2002. 13:395–400.
3. Pierre M. Sur deux cas d'acromegalie. Hypertrophe singluliere no congenitale des extremites superieures, inferieures et cephalique. Rev Med Fran. 1886. 6:297–333.
4. Wass JA. Growth hormone, insulin-like growth factor-I and its binding proteins in the follow-up of acromegaly. J Endocrinol. 1997. 155:S17–S19.
5. Jenkins PJ, Besser M. Clinical perspective: acromegaly and cancer: a problem. J Clin Endocrinol Metab. 2001. 86:2935–2941.
6. Webb SM, Casanueva F, Wass JA. Oncological complications of excess GH in acromegaly. Pituitary. 2002. 5:21–25.
7. Orme SM, McNally RJ, Cartwright R, Belchetz PE. Mortality and cancer incidence in acromegaly: a retrospective cohort study. J Clin Endocrinol Metab. 1998. 83:2730–2934.
8. Lin B, Kinoshita Y, Hato F, Tsuji Y. Enhancement of DNA synthetic activity of thymic lymphocytes by the culture supernatant of thymic epithelial cells stimulated by growth hormone. Cell Mol Biol. 1997. 43:351–359.
9. LeRoith D, Baserga R, Helman L, Roberts C. Insulin-like growth factors and cancer. Ann Intern Med. 1995. 122:54–59.
10. Yamaguchie M, Tate G, Yoshizawa Y, Midorikawa T, Sanada Y, Kumada K. Hepatocellular carcinoma in a patient with acromegaly and high serum levels of insulin-like growth factor I: report of a case. Surg Today. 2002. 32:1008–1011.
11. Moon HD, Simpson ME, Li CH, Evans HM. Neoplasia in rats treated with pituitary growth hormone I, pulmonary and lymphatic tissues. Cancer Res. 1950. 14:297–308.
12. Resnicoff M, Li W, Basak S, Herlyn D, Baserga R, Rubin R. Inhibition of rat C6 glioblastoma tumor growth by expression of insulin-like growth factor-1 receptor antisense mRNA. Cancer Immunol Immunother. 1996. 42:64–68.
13. Melmed S. Acromegaly and cancer: not a problem? J Clin Endocrinol Metab. 2001. 86:2929–2934.
14. Jenkins PJ, Bustin SA. Evidence for a link between IGF-I and cancer. European J Endocrinol. 2004. 151:S17–S22.
15. Sell C, Dumenil G, Deveaud C, Miura M, Coppola D, DeAngelis T, Rubin R, Efstratiadis A, Baserga R. Effect of a null mutation of the type 1 IGF receptor gene on growth and transformation of mouse embryo fibroblasts. Mol Cell Biol. 1994. 14:3604–3612.
16. Sell C, Rubini M, Rubin R, Liu JP, Efstratiadis A, Baserga R. Simian virus 40 large tumor antigen is unable to transform mouse embryonic fibroblasts lacking type-1 insulin-like growth factor receptor. Proc Natl Acad Sci USA. 1993. 90:11217–11221.
17. Mauro L, Surmacz E. IGF-1 receptor, cell-cell adhesion, tumour development and progression. J Mol Histol. 2004. 35:247–253.
18. Baris M, Gridley G, Ron E, Weiderpass E, Mellemkhaer L, Ekbom A, Olser JH, Baron JA, Fraumeni JF Jr. Acromegaly and cancer risk: a cohort study in Sweden and Denmark. Cancer Cause Control. 2002. 13:395–400.
19. Klein I, Parveen G, Gavaler JS, Vanthiel DH. Colonic polyps in patients with acromegaly. Ann Intern Med. 1982. 97:27–30.
20. Balkany C, Cushing GW. An association between acromegaly and thyroid carcinoma. Thyroid. 1995. 5:47–50.
21. Cheung NW, Boyages SC. Increased incidence of neoplasia in females with acromegaly. Clin Endocrinol (Oxf). 1997. 47:323–327.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download