Abstract
Background
Activation of G-protein coupled-somatostatin receptors induces the release of calcium from inositol 1, 4, 5-trisphosphate-sensitive intracelluar stores. G-protein-coupled receptor signaling decreases with prolonged exposure to an agonist.
Subjects and Methods
Fura-2-based digital Ca2+ imaging was used to study the effects of prolonged exposure to an agonist on the somatostatin-induced intracellular Ca2+ concentration ([Ca2+]i) increases in NG108-15 cells, which were differentiated with CO2-independent medium and 10 µM forskolin.
Results
Exposure to somatostatin (1 µM) for 30 min completely desensitized the NG108-15 cells to a second somatostatin-induced response. The cells recovered gradually over 20 min following washout of the somatostatin. The desensitization was not due to depletion of the intracellular Ca2+ stores, and pretreatment for 30 min with bradykinin (100 nM), which activates phospholipase C, or DADLE (D-Ala2-D-Leu5 enkephalin, 1 µM), which activates phospholipase C, failed to cross-desensitize the somatostatin-evoked [Ca2+]i increases. Treatment with 8-cpt-cAMP (0.1 mM) for 30 min did not influence the somatostatin-induced [Ca2+]i increases. Phorbol 12, 13-dibutyrate (PdBu, 1 µM) blocked the response completely. Down-regulation of PKC due to 24 h exposure of PdBu (1 µM) inhibited the somatostatin-induced desensitization.
Figures and Tables
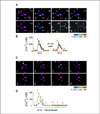 | Fig. 1Prolonged exposure to somatostatin desensitizes somatostatin-induced [Ca2+]i increase in NG108-15 cells. (A) Pseudocolor representations of [Ca2+]i were derived from fura-2-based digital images. Somatostatin-induced [Ca2+]i transients were elicited by superfusion with 1 µM somatostatin for 90s at 25 min intervals in this field. Responses from the three cells indicated by arrows in A, frame 1 are plotted in B. Somatostatin was applied as indicated by the horizontal bars. The numbers along the plot refer to the frame numbers in A. (C) Pseudocolor images show that Somatostatin responses were completely desensitized following a 30 min exposure to Somatostatin (1 µM). Responses from the three cells indicated by arrows in C, frame 1 are plotted in D. Somatostatin was applied continuously as indicated by the horizontal bar. Gaps in the recording (during which the cells were not illuminated) are indicated by diagonal lines. The numbers along the plot refer to the frame numbers in C |
 | Fig. 2Somatostatin-induced [Ca2+]i increases displayed a graded recovery from desensitization in NG108-15 cells. (A-D) Representative traces show recovery from 30 min exposure to somatostatin (1 µM) following wash periods of various duration. NG108-15 cells were washed with Hepes Hank's buffer (A) 2, (B) 6, (C) 10, or (D) 20 min prior to a second challenge with somatostatin (1 µM). (E) Summary plot shows the time course of recovery from desensitization for the wash times presented in A-D. Data are means +/- SE of at least 9 cells. |
 | Fig. 3No cross desensitization between somatostatin (SM)-and bradykinin (BK, Gq-coupled receptor agonist)-induced responses in NG108-15 cells. (A) Bradykinin (100 nM)-evoked [Ca2+]i increases were not affected by 30 min exposure to 1 µM somatostatin. (B) Somatostatin (1 µM)-evoked responses were not affected by 30 min exposure to 100 nM bradykinin. |
 | Fig. 4No cross desensitization between somatostatin (SM)-and DADLE (δ-opioid receptor agonist, Gi-coupled receptor agonist)-induced responses. (A) DADLE (1 µM)-evoked [Ca2+]i increases were not affected by prolonged exposure to 1 µM somatostatin. (B) Somatostatin (1 µM)-evoked responses were not affected by 30 min exposure to 1 µM DADLE. |
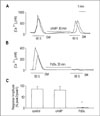 | Fig. 5Effects of cAMP and PdBu on somatostatin-induced [Ca2+]i increase in NG108-15 cells. Cells were treated for 30 min prior to and during the second somatostatin application with (A) 0.1 mM 8-bromo-cAMP or (B) 1 µM PdBu. (C) Histogram summarizes responses normalized to initial control response for control (n=29), cAMP-treated (n=5), and PdBu-treated cells (n=7). Data are means +/- SE. *P< 0.01 relative to control and cAMP. |
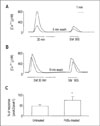 | Fig. 6Effects of chronic PdBu treatment on somatostatin-induced [Ca2+]i increase in NG108-15 cells. NG108-15 cells were grown in the presence of 1 µM PdBu for 24h. (A) Somatostatin (1 µM)-evoked responses were desensitized following a 30 min exposure of somatostatin (1 µM) and a 6 min wash in untreated cells. (B) Somatostatin-induced desensitization inhibited by chronic PdBu treatment. (C) Histogram summarizes responses normalized to initial control response for untreated cells (n=36) and chronic PdBu-treated cells (n= 5). *P< 0.05 relative to untreated. |
References
1. Hagen PM. Somatostatin receptor expression in clinical immunology. Metabolism. 1996. 45:86–87.
2. Schindler M, Humphrey PPA, Emson PC. Somatostatin receptors in the central nervous system. Prog Neurobiol. 1996. 50:9–47.
3. Schonbrunn A, Gu A-Z, Dournard P, Beaudet A, Tannenbaum GS, Brown PJ. Somatostatin receptor subtypes: specific expression and signaling properties. Metabolism. 1996. 45:8–11.
4. Hoyer D, Bell GI, Berelowitz M, Epelbaum J, Feniuk W, Humphrey PP, O'Carroll AM, Patel YC, Schonbrunn A, Taylor JE, Reisine T. Classification and nomenclature of somatostatin receptors. Trends Pharmacol Sci. 1995. 16:86–88.
5. Yasuda K, Rens-Dominano S, Breder CD, Law SF, Saper CB, Reisine T, Bell GI. Cloning of a novel SRIF receptor, SSTR3, that is coupled to adenylyl cyclase. J Biol Chem. 1992. 267:20422–20428.
6. Vanetti M, Vogt G, Holt V. The isoforms of the mouse somatostatin receptor (MSSTR2A and MSSTR2B) differ in coupling efficiency to adenylate- cyclase in agonist-induced receptor desensitization. FEBS Lett. 1993. 331:260–266.
7. Liapakis G, Tallent M, Reisine T. Molecular and functional properties of somatostatin receptor subtypes. Metabolism. 1996. 45:12–13.
8. Beaumont V, Hepworth MB, Luty JS, Kelly E, Henderson G. Somatostatin receptor desensitization in NG108-15 cells. J Biol Chem. 1998. 273:33174–33183.
9. Jin W, Lee NM, Loh HH, Thayer SA. Opioids mobilize calcium from inositol 1,4,5-trisphosphate-sensitive stores in NG108-15 cells. J Neurosci. 1994. 14:1920–1929.
10. Smart D, Lambert DG. δ-opioids stimulate inositol 1,4,5-trisphosphate formation, and so mobilize Ca2+ from intracelluar stores, in undifferented NG108-15 cells. J Neurochem. 1996. 66:1462–1467.
11. Akbar M, Okajima F, Tomura H, Majid MA, Yamada Y, Seino S, Kondo Y. Phospholipase C activation and Ca2+ mobilization by cloned human somatostatin receptor subtypes 1-5, in transfected COS-7 cells. FEBS Lett. 1994. 348:192–196.
12. Kubota A, Yamada Y, Kagimoto S, Yasuda K, Someya Y, Ihara Y, Okamoto Y, Kozasa T, Seino S, Seino Y. Multiple effector coupling of somatostatin receptor subtype SSTR1. Biochem Biophys Res Comm. 1994. 204:176–186.
13. Tomura H, Okajima F, Akbar M, Abdulmajid M, Sho K, Kondo Y. Transfected human somatostatin receptor type 2, SSTR2, not only inhibits adenylate cyclase but also stimulates phospholipase C and Ca2+ mobilization. Biochem Biophys Res Comm. 1994. 200:986–992.
14. Rhie D-J, Sung JH, Ha US, Ha U-S, Kim HJ, Min DS, Hahn SJ, Kim MS, Jo YH, Yoon SH. Somatostatin mobilizes calcium from inositol 1,4,5-trisphosphate-sensitive stores in NG108-15 cells. Brain Res. 2003. 975:120–128.
15. Freedman NJ, Lefkowitz RJ. Desensitization of G protein-coupled receptors. Rec Prog Hormone Res. 1996. 51:319–335.
16. Grynkiewicz G, Peonie M, Tsien RY. A new generation of calcium indicators with greatly improved fluorescence properties. J Biol Chem. 1985. 260:3440–3450.
17. Yoon SH, Lo T-M, Loh HH, Thayer SA. δ-opioid-induced liberation of Gβγ mobilizes Ca2+ stores in NG108-15 cells. Mol Pharmacol. 1999. 56:902–908.
18. Nishizuka Y. Protein kinases in signal transduction. Trends Biochem Sci. 1984. 163–169.
19. Lohse MJ. Molecular mechanisms of membrane receptor desensitization. Biochim Biophys Acta. 1993. 1179:171–188.
20. Yoon SH, Jin WZ, Spencer RJ, Loh HH, Thayer SA. Desensitization of δ-opioid-induced mobilization of Ca2+ stores in NG108-15 cells. Brain Res. 1998. 802:9–18.
21. Lo T-M, Thayer SA. Refilling the inositol 1,4,5-trisphosphate-sensitive store in neuroblastoma X glioma hybrid NG108-15 cells. Am J Physiol: Cell Physiol. 1993. 33:C641–C653.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download