Abstract
Backgrounds
GH3 cells lack growth hormone (GH)-releasing hormone (GHRH) receptors. In this study, GH3 cells permanently transfected with human GHRH receptor cDNA (GH3-GHRHR cells), were established in order to examine the effects of GHRH and G protein mutation (gsp oncogene) on the levels of somatostatin receptor mRNA.
Methods
GH3 cells were permanently transfected with a plasmid expressing human GHRH receptor cDNA. The GHRH receptor mRNA was detected by RT-PCR. The responsiveness to GHRH was evaluated using a GHRH binding assay, Western blot analysis, Northern blot analysis, and measurements of the intracellular cAMP levels and GH release. Cells were transiently transfected with the gsp oncogene, and then treated with GHRH or octreotide for 4h. The sst1 and sst2 mRNA levels were measured using real-time RT-PCR analyses.
Results
GHRH receptor mRNA was detected in the GH3 cells permanently transfected with human GHRH receptor cDNA. The GHRH binding assay showed that GHRH was bound to the GH3-GHRHR cells. The GHRH treatment increased the intracellular cAMP levels, GH release, GH mRNA levels, and MAPK activity, as well as the levels of sst1 and sst2 mRNA. Transient expression of the gsp oncogene for 48h increased the cAMP, GH release, and levels of sst1 and sst2 mRNA. In the gsp-transfected GH3-GHRHR cells, GHRH stimulation resulted in decreases in the magnitude of the increase in the levels of sst1 and sst2 mRNA compared to those transfected with a control vector. Octreotide treatment did not alter the levels of sst1 and sst2 mRNA in either the control or gsp-transfected cells.
Conclusion
These results suggest that GH3 cells permanently transfected with the GHRH receptor are useful in the in vitro studies on the actions of GHRH. The gsp oncogene was shown to increases the levels of sst1 and sst2 mRNA in GH3 cells, but these findings are unlikely to be the major mechanism by which gsp-positive pituitary tumors show a greater response to somatostatin. The discrepancy between the in vivo and these in vitro results should be examined further.
Figures and Tables
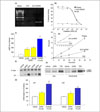 | Fig. 1Characteristics of GH3 cells permanently transfected with human GHRH receptor cDNA (GH3-GHRHR cells).
(A) RT-PCR analysis of GHRH receptor mRNA in wild-type GH3 and GH3-GHRHR cells. GHRH receptor mRNA was only detected in GH3-GHRHR cells. (B) Binding assay of GHRH to GHRH receptors in wild-type GH3, GH3-GHRHR, and primary cultured rat anterior pituitary cells. 125I-Labeled and increasing concentrations of unlabeled hGHRH (1-44) amide were added to cells. Results are expressed as a percentage of the maximum specific binding. (C) Intracellular cAMP levels in response to GHRH (10, 100 nM) and forskolin (10 µM). (D) GH response to GHRH (10 nM) in wild-type GH3, GH3-GHRHR, and primary pituitary cells. GH levels were measured 15, 30, and 60 min after adding GHRH. (E) Effects of GHRH (10 nM) and dexamethasone (10 µM) on GH mRNA levels in GH3-GHRHR cells. Cells were treated with GHRH and dexamethasone for 72h. GH mRNA levels were measured by Northern blot assay. (F) MAPK activation in GH3-GHRHR cells. Western blot analysis was performed using rabbit anti-phospho-p44/p42 MAPK. TRH was used as a positive control. (G) Effects of GHRH (10 nM) and forskolin (10 µM) on sst1 and sst2 mRNA levels in GH3-GHRHR cells. Cells were incubated with GHRH and forskolin for 4h. Sst1 and sst2 mRNA levels were measured by real-time RT-PCR. *, P < 0.05, **, P < 0.01.
|
 | Fig. 2Effect of transient transfection of gsp oncogenes into GH3-GHRHR cells on intracellular cAMP levels. Cells were transfected with pSV2, pSV2-wt (wt), pSV2-αs-R201H (201), and pSV2-αs-Q227L (227) and incubated for 24h, 48h, and 72h. Intracellular cAMP levels were measured by RIA. Results are expressed as a percent of pSV2 controls (Mean±S.E.; n=4 wells from two independent experiments). *, P < 0.05, **, P < 0.01. |
 | Fig. 3Effect of transient transfection of gsp oncogenes into GH3-GHRHR cells on GH release. Cells were transfected with pSV2, pSV2-wt (wt), pSV2-αs-R201H (201), and pSV2-αs-Q227L (227) and incubated for 48h. GH levels were measured by RIA. Data are expressed as Mean±S.E. (n=4 wells from two independent experiments). *, P < 0.05. |
 | Fig. 4Effect of transient transfection of gsp oncogenes into GH3-GHRHR cells on sst1 and sst2 mRNA levels. Cells were transfected with pSV2, pSV2-wt (wt), pSV2-αs-R201H (201), and pSV2-αs-Q227L (227) and incubated for 48h. Sst1 and sst2 mRNA levels were measured by real-time RT-PCR assay. Receptor mRNA levels were adjusted by β-actin and expressed as a percent of pSV2 controls (Mean±S.E.; n=4 wells from two independent experiments). *, P < 0.05. |
 | Fig. 5Effects of GHRH and octreotide stimulation on sst1 and sst2 mRNA levels in GH3-GHRHR cells transfected with pSV2, pSV2-wt (wt), pSV2-αs-R201H (201), and pSV2-αs-Q227L (227). Transfected cells were incubated for 48h and stimulated with GHRH (10 nM) and octreotide (10 nM) for 4h. Sst1 and sst2 mRNA levels were measured by real-time RT-PCR assay. Receptor mRNA levels were adjusted by β-actin and expressed as a percent of pSV2 controls (Mean±S.E.; n=4 wells from two independent experiments). *, P < 0.05, **, P < 0.01. |
References
1. Hall R, Besser GM, Schally AV, Coy DH, Evered D, Goldie DJ, Kastin AJ, McNeilly AS, Mortimer CH, Phenekos C, Tunbridge WM, Weightman D. Action of growth-hormone-release inhibitory hormone in healthy men and in acromegaly. Lancet. 1973. 2:581–584.
2. Yen SS, Siler TM, Devane GW. Effect of somatostatin in patients with acromegaly. Suppression of growth hormone, prolactin, insulin and glucose levels. N Engl J Med. 1974. 290:935–938.
3. Oppizzi G, Botalla L, Verde G, Cozzi R, Liuzzi A, Chiodini PG. Homogeneity in the growth hormone-lowering effect of dopamine andsomatostatin in acromegaly. J Clin Endocrinol Metab. 1980. 51:616–619.
4. Pieters GF, Romeijn JE, Smals AG, Kloppenborg PW. Somatostatin sensitivity and growth hormone responses to releasing hormones and bromocryptine in acromegaly. J Clin Endocrinol Metab. 1982. 54:942–948.
5. Lamberts SWJ, Uitterlinden P, Verschoor L, van Dongen KJ, Del Pozo E. Long-term treatment of acromegaly with the somatostatin analogue SMS 20l-995. N Engl J Med. 1985. 313:1576–1580.
6. Lamberts SW, Oosterom R, Neufeld M, Del Pozo E. The somatostatin analog SMS 201-995 induces long-acting inhibition of growth hormone secretion without rebound hypersecretion in acromegalic patients. J Clin Endocrinol Metab. 1985. 60:1161–1165.
7. Lamberts SW, Zweens M, Verschoor L, Del Pozo E. A comparison among the growth hormone-lowering effects in acromegaly of the somatostatin analog SMS 201-995, bromocriptine, and the combination of both drugs. J Clin Endocrinol Metab. 1986. 63:16–19.
8. Jackson IM, Barnard LB, Lamberton P. Role of a long-acting somatostatin analogue (SMS 201-995) in the treatment of acromegaly. Am J Med. 1986. 81:94–101.
9. Chiodini PG, Cozzi R, Dallabonzana D, Oppizzi G, Verde G, Petroncini M, Liuzzi A, Del Pozo E. Medical treatment of acromegaly with SMS 201-995, a somatostatin analog: a comparison with bromocriptine. J Clin Endocrinol Metab. 1987. 64:447–453.
10. Ho KY, Weissberger AJ, Marbach P, Lazarus L. Therapeutic efficacy of the somatostatin analog SMS 201-995 (octreotide) in acromegaly. Ann Intern Med. 1990. 112:173–181.
12. Ikuyama S, Nawata H, Kato K-I, Ibayashi H, Nakagaki H. Plasma growth hormone responses to somatostatin (SRIH) and SRIH receptors in pituitary adenomas in acromegalic patients. J Clin Endocrinol Metab. 1986. 62:729–733.
13. Reubi JC, Landolt AM. The growth hormone responses to octreotide in acromegaly correlate with adenoma somatostatin receptor status. J Clin Endocrinol Metab. 1989. 68:844–850.
14. Bertherat J, Chanson P, Dewailly D, Dupuy M, Jaquet P, Peillon F, Epelbaum J. Somatostatin receptors, adenylate cyclase activity, and growth hormone (GH) response to octreotide in GH-secreting adenomas. J Clin Endocrinol Metab. 1993. 77:1577–1583.
15. Yamada Y, Post SR, Wang K, Tager HS, Bell GI, Seino S. Cloning and functional characterization of a family of human and mouse somatostatin receptors expressed in brain, gastrointestinal tract, and kidney. Proc Natl Acad Sci USA. 1992. 89:251–255.
16. Kluxen F, Bruns C, Lubbert H. Expression cloning of a rat brain somatostatin receptor cDNA. Proc Natl Acad Sci USA. 1992. 89:4618–4622.
17. Bruno JF, Xu Y, Song J, Berelowitz M. Molecular cloning and functional expression of a brain-specific somatostatin receptor. Proc Natl Acad Sci USA. 1992. 89:11151–11155.
18. Yamada Y, Reisine T, Law SF, Ihara Y, Kubota A, KAgimoto S, Seino M, Seino Y, Bell GI, Seino S. Somatostatin receptors, an expanding gene family: cloning and functional characterization of human SSTR3, a protein coupled to adenylyl cyclase. Mol Endocrinol. 1992. 6:2136–2142.
19. O'Carroll A-M, Loliat SJ, Konig M, Mahan LC. Molecular cloning and expression of a pituitary somatostatin receptor with preferential affinity for somatostatin28. Mol Pharmacol. 1992. 42:939–946.
20. Greenman Y, Melmed S. Expression of three somatostatin receptor subtypes in pituitary adenomas: evidence for preferential SSTR5 expression in the mammosomatotroph lineage. J Clin Endocrinol Metab. 1994. 79:724–729.
21. Greenman Y, Melmed S. Heterogeneous expression of two somatostatin receptor subtypes in pituitary tumors. J Clin Endocrinol Metab. 1994. 78:398–403.
22. Miller GM, Alexander JM, Bikkal HA, Katznelson L, Zervas NT, Klibanski A. Somatostatin receptor subtypegene expression in pituitary adenomas. J Clin Endocrinol Metab. 1995. 80:1386–1392.
23. Patel YC, Srikant CB. Subtype selectivity of peptide analogs for all five cloned human somatostatin receptors (hsstr 1-5). Endocrinology. 1994. 135:2814–2817.
24. Yang I, Park S, Ryu M, Woo J, Kim S, Kim J, Kim Y, Choi Y. Characteristics of gsp-positive growth hormone-secreting pituitary tumors in Korean acromegalic patients. Eur J Endocrinol. 1996. 134:720–726.
25. Spada A, Arosio M, Bochicchio D, Bazzoni N, Vallar L, Bassetti M, Faglia G. Clinical, biochemical, and morphological correlates in patients bearing growth hormone-secreting pituitary tumors with or without constitutively active adenylyl cyclase. J Clin Endocrinol Metab. 1990. 71:1421–1426.
26. Patel YC, Greenwood M, Kent G, Panetta R, Srikant CB. Multiple gene transcript of the somatostatin receptor SSTR2: Tissue selective distribution and cAMP regulation. Biochem Biophys Res Commun. 1993. 192:288–294.
29. Park S, Kamegai J, Johnson TA, Frohman LA, Kineman RD. Modulation of pituitary somatostatin receptor subtype (sst1-5) messenger ribonucleic acid levels by changes in the growth hormone axis. Endocrinology. 2000. 141:3556–3530.
31. Barlier A, Pellegrini-Bouiller I, Gunz G, Zamora AJ, Jaquet P, Enjalbert A. Impact of gsp oncogene on the expression of genes coding for Gs alpha, Pit-1, Gi alpha, and somatostatin receptor 2 in human somatotroph adenomas: involvement in ortreotide sensitivity. J Clin Endocrinol Metab. 1999. 84:2759–2765.
32. Zeytin FN, Gick GG, Brazeau P, Ling N, Mclaughlin M, Bancroft C. Growth hormone (GH)-releasing factor does not regulate GH rlease or GH mRNA levels in GH3 cells. Endocrinology. 1984. 114:2054–2059.
33. Park S, Sohn S, Kineman RD. Fasting-induced changes in the hypothalamic-pituitary-GH axis in the absence of GH expression: lessons from the spontaneous dwarf rat. J Endocrinol. 2004. 180:369–378.
35. Perez FR, Casabiell X, Camina JP, Zugaza JL, Casanueva FF. cis-unsaturated free fatty acids block growth hormone and prolactin secretion in thyrotropin-releasing hormone-stimulated GH3 cells by perturbing the function of plasma membrane integral proteins. Endocrinology. 1997. 138:264–272.
36. Bruno JF, Xu Y, Berelowitz M. Somatostatin regulates somatostatin receptor subtype mRNA expression in GH3 cells. Biochem Biophys Res Commun. 1994. 202:1738–1743.
37. Cassel D, Selinger Z. Activation of turkey erythrocyte adenylate cyclase and blocking of the catecholamine-stimulated GTPase by guanosine 5'-(gamma-thio) triphosphate. Biochem Biophys Res Commun. 1977. 77:868–873.
38. Der CJ, Finkel T, Cooper GM. Biological and biochemical properties of human rasH genes mutated at codon 61. Cell. 1986. 44:167–176.
39. Landis CA, Masters SB, Spada A, Pace AM, Bourne HR, Vallar L. GTPase inhibiting mutations activate the alpha chain of Gs and stimulate adenylyl cyclase in human pituitary tumours. Nature. 1989. 340:692–696.
40. Gaiddon C, Boutillier AL, Monnier D, Mercken L, Loeffler JP. Genomic effects of the putative oncogene Gs-α. Chronic transcriptional activation of the c-fos proto-oncogene in endocrine cells. J Biol Chem. 1994. 269:22663–22671.
41. Rohrer SP, Hayes E, Berk SC, Hutchins SM, Shen DM, Xiong Y, Parma R, Foor P, Mitra SW, Degrado SJ, Shu M, Klopp JM, Cai SJ, Blake A, Chan WW, Pasternak A, Yang L, Patchett A, Smith RG, Chapman KT, Schaeffer JM. Rapid identification of subtype-selective agonists of the somatostatin receptor through combinatorial chemistry. Science. 1998. 282:737–740.
42. Shimon I, Taylor JE, Dong JZ, Bitonte RA, Kim S, Morgan B, Coy DH, Culler MD, Melmed S. Somatostatin receptor subtype specificity in human fetal pituitary cultures. Differential role of SSTR2 and SSTR5 for growth hormone, thyroid-stimulating hormone, and prolactin regulation. J Clin Invest. 1997. 99:789–798.
43. Kreienkamp H-J, Akgűn E, Baumeister H, Meyerhof W, Richter D. Somatostatin receptor subtype 1 modulates basal inhibition of growth hormone release in somatotrophs. FEBS Lett. 1999. 462:464–466.
44. Day R, Dong W, Panetta R, Kracier J, Greenwood MT, Patel YC. Expression of mRNA for somatostatin receptor (sstr) types 2 and 5 in individual rat pituitary cells. A double labeling in situ hybridization analysis. Endocrinology. 1995. 136:5232–5235.
45. Kumar U, Laird D, Srikant CB, Escher E, Patel YC. Expression of the five somatostatin receptor (SSTR15) subtypes in rat pituitary somatotropes: quantitative analysis by double - label immunoflourescence confocal microscopy. Endocrinology. 1997. 138:4473–4476.
46. Mezey E, Hunyady B, Mitra S, Hayes E, Liu Q, Schaeffer J, Schonbrunn A. Cell specific expression of the sst2A and sst5 somatostatin receptors in the rat anterior pituitary. Endocrinology. 1998. 139:414–419.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download