Figures and Tables
Fig. 1
Colocalization of prolactin (PRL) with stem cell markers in livers of mice treated with Ad-Pit-1. Paraffin sections of livers of mice at day 5 after treatment were analyzed by double immunofluorescence staining for PRL with c-kit, thy-1, or cytokeratin 14. Cells with red fluorescent cytoplasm express PRL (yellow arrow in left panel) and cells with green fluorescent cytoplasm (red arrow in center panel) express c-kit (upper), thy-1 (middle) or cytokeratin 14 (lower). PV: Portal vein. Upper and middle panels are 630 × magnification and lower panel is 1000 × magnification. Lactotrope cells express hepatic stem cells marker at the initial stage of differentiation. Reprinted from (8) with permission.
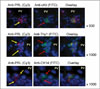
Fig. 2
Adenoviral vector and Ad/AAV hybrid vectors carrying gene of interest. Infection of cells with Ad/AAV hybrid vector enables precise excision of the AAV inverted terminal repeat (ITR)-flanked gene from adenoviral genome, subsequently integration into the host genome occurs. In the presence of Rep78 expression unit, transgene is predictably integrated into the AAVS1 locus on human chromosome 19.

Fig. 3
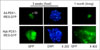
GFP expression in HepG2 cells infected with adenovirus or Ad/AAV hybrid vectors carrying pancreas-duodenum homeobox 1 (PDX1)-internal ribosomal entry site (IRES)-green fluorescent protein (GFP). GFP was more abundantly expressed in HepG2 cells infected with Ad/AAV hybrid vector. (not published data)
References
1. Yamaoka T. Regeneration therapy of pancreatic beta cells: towards a cure for diabetes? Biochem Biophys Res Commun. 2002. 296:1039–1043.
2. Matsumoto T, Yamaguchi M, Kuzume M, Matsumiya A, Kumada K. Insulin gene transfer with adenovirus vector via the spleen safely and effectively improves posthepatectomized conditions in diabetic rats. J Surg Res. 2003. 110:228–234.
3. Lee HC, Kim SJ, Kim KS, Shin HC, Yoon JW. Remission in models of type 1 diabetes by gene therapy using a single-chain insulin analogue. Nature. 2000. 408:483–488.
4. Ferber S, Halkin A, Cohen H, Ber I, Einav Y, Goldberg I, Barshack I, Seijffers R, Kopolovic J, Kaiser N, Karasik A. Pancreatic and duodenal homeobox gene 1 induces expression of insulin genes in liver and ameliorates streptozotocin-induced hyperglycemia. Nat Med. 2000. 6:568–572.
5. Kojima H, Fujimiya M, Matsumura K, Younan P, Imaeda H, Maeda M, Chan L. NeuroD-betacellulin gene therapy induces islet neogenesis in the liver and reverses diabetes in mice. Nat Med. 2003. 9:596–603.
6. Zhang Y, Bai XF, Huang CX. Hepatic stem cells: existence and origin. World J Gastroenterol. 2003. 9:201–204.
7. Falkowski O, An HJ, Ianus IA, Chiriboga L, Yee H, West AB, Theise ND. Regeneration of hepatocyte 'buds' in cirrhosis from intrabiliary stem cells. J Hepatol. 2003. 39:357–364.
8. Lee EJ, Russell T, Hurley L, Jameson JL. Pit-1 induces transient differentiation of adult hepatic stem cells into prolactin-producing cells in vivo. Mol Endocrinol. 2005. 19:964–971.
9. Zajicek G, Ariel I, Arber N. The streaming liver. III. Littoral cells accompany the streaming hepatocyte. Liver. 1988. 8:213–218.
10. Arber N, Zajicek G, Ariel I. The streaming liver. II. Hepatocyte life history. Liver. 1988. 8:80–87.
11. Drolet DW, Scully KM, Simmons DM, Wegner M, Chu KT, Swanson LW, Rosenfeld MG. TEF, a transcription factor expressed specifically in the anterior pituitary during embryogenesis, defines a new class of leucine zipper proteins. Genes Dev. 1991. 5:1739–1753.
12. Godfrey P, Rahal JO, Beamer WG, Copeland NG, Jenkins NA, Mayo KE. GHRH receptor of little mice contains a missense mutation in the extracellular domain that disrupts receptor function. Nat Genet. 1993. 4:227–232.
13. Glick E, Leshkowitz D, Walker MD. Transcription factor BETA2 acts cooperatively with E2A and PDX1 to activate the insulin gene promoter. J Biol Chem. 2000. 275:2199–2204.
14. Aramata S, Han SI, Yasuda K, Kataoka K. Synergistic activation of the insulin gene promoter by the beta-cell enriched transcription factors MafA, Beta2, and Pdx1. Biochim Biophys Acta. 2005. 1730:41–46.
15. Kaneto H, Nakatani Y, Miyatsuka T, Matsuoka TA, Matsuhisa M, Hori M, Yamasaki Y. PDX-1/VP16 fusion protein, together with NeuroD or Ngn3, markedly induces insulin gene transcription and ameliorates glucose tolerance. Diabetes. 2005. 54:1009–1022.
16. Schiedner G, Morral N, Parks RJ, Wu Y, Koopmans SC, Langston C, Graham FL, Beaudet AL, Kochanek S. Genomic DNA transfer with a high-capacity adenovirus vector results in improved in vivo gene expr ession and decreased toxicity. Nat Genet. 1998. 18:180–183.
17. Surosky RT, Urabe M, Godwin SG, McQuiston SA, Kurtzman GJ, Ozawa K, Natsoulis G. Adeno-associated virus Rep proteins target DNA sequences to a unique locus in the human genome. J Virol. 1997. 71:7951–7959.
18. Sun BD, Chen YT, Bird A, Amalfitano A, Koeberl DD. Long-term correction of glycogen storage disease type II with a hybrid Ad-AAV vector. Mol Ther. 2003. 7:193–201.
19. Samulski RJ, Sally M, Muzyczka N. Friedmann T, editor. Adeno-associate viral vectors. In The development of human gene therapy. 1999. Cold Spring harbor: Cold Spring Harbor Laboratory Press;131–171.
20. Buning H, Nicklin SA, Perabo L, Hallek M, Baker AH. AAV-based gene transfer. Curr Opin Mol Ther. 2003. 5:367–375.
21. Carter PJ, Samulski RJ. Adeno-associated viral vectors as gene delivery vehicles. Int J Mol Med. 2000. 6:17–27.
22. Samulski RJ, Zhu X, Xiao X, Brook JD, Housman DE, Epstein N, Hunter LA. Targeted integration of adeno-associated virus (AAV) into human chromosome 19. Embo J. 1991. 10:3941–3950.
23. Recchia A, Parks RJ, Lamartina S, Toniatti C, Pieroni L, Palombo F, Ciliberto G, Graham FL, Cortese R, La Monica N, Colloca S. Site-specific integration mediated by a hybrid adenovirus/adeno-associated virus vector. Proc Natl Acad Sci U S A. 1999. 96:2615–2620.
24. Lieber A, Steinwaerder DS, Carlson CA, Kay MA. Integrating adenovirus-adeno-associated virus hybrid vectors devoid of all viral genes. J Virol. 1999. 73:9314–9324.
25. Douin V, Bornes S, Creancier L, Rochaix P, Favre G, Prats AC, Couderc B. Use and comparison of different internal ribosomal entry sites (IRES) in tricistronic retroviral vectors. BMC Biotechnol. 2004. 4:16.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download