Abstract
Purpose
This analysis was conducted to evaluate the cost-effectiveness of gemcitabine-cisplatin chemotherapy for non small-cell lung cancer patients in an outpatient setting compared with the traditional inpatient setting.
Methods
A cost-effective analysis was conducted from a societal perspective. The effects of treatment, which was measured as an adverse event rate, were abstracted from a published literature search and empirical data from one university hospital. The costs included both direct and indirect costs. Direct costs included hospitalizations, outpatient visits, and lab tests. Pharmaceutical costs were excluded in analysis because they were same for both options. Indirect costs included productivity loss of patients as well as care-givers. In order to determine the robustness of the results, sensitivity analysis on treatment protocol was conducted.
Results
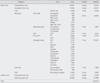
Literature search showed no difference in adverse effect rates between inpatient treatment protocol and outpatient treatment protocol. Therefore, this analysis is a cost-minimization analysis. Cost-savings in the outpatient setting was 555,936 won for one treatment cycle. Our sensitivity analysis indicated that the outpatient chemotherapy still showed cost-savings, regardless of changes in treatment protocol.
Figures and Tables
References
1. Abratt RP, Bezwoda WR, Falkson G, Goedhals L, Hacking D, Rugg TA. Efficacy and safety profile of gemcitabine in non-small-cell lung cancer: A phase II study. Journal of Clinical Oncology. 1994. 12:1535–1540.

2. Anderson H, Lund B, Bach F, Thatcher N, Walling J, Hansen HH. Single-agent activity of weekly gemcitabine in advanced non-small-cell lung cancer: A Phase II study. Journal of Clinical Oncology. 1994. 12:1821–1826.

3. Bischoff HG, Eberhardt W, Wilke H, Gatzemeier U. Experiences with gemcitabine in advanced non-small-cell lung cancer (NSCLC): Report of a phase-1 dose-finding study of gemcitabine and ifosphamide. Canadian Journal of Infectious Disease. 1995. 6:89.
4. Chang HJ, Ahn JB, Lee JG, Shim KY, Rha SY, Kim SK, et al. Efficacy of gemcitabine chemotherpy in advanced Non-Small-Cell Lung Cancer (NSCLC): A phase 2 study. Journal of Korean Cancer Association. 1999. 31:523–533.
5. Holmes S. The oral complications of specific anti-cancer therapy. International Journal of Nursing Studies. 1991. 28:343–360.

6. Kim SK. Cost of non-small-cell lung cancer. Dailymedi. 2004. Retrieved October 20, 2006. from http://www.dailymedi.com/news/opdb/index.php?cmd=view&dbt=article&code=41040&page=2&sel=ticon&key=¡™¿?&cate=&rgn=&term.
7. Kim SJ, Yi MS, Eun Y, Ko MH, Kim JH, Kim DO, et al. Role-identity of home care nurse practitioners. Journal of Korean Academy of Nursing. 2006. 36:103–113.

8. Cause of death statistics. Korea National Statistical Office. 2006. Retrieved October 20, 2006. from http://www.kosis.kr.
9. Annual report of the Korea central cancer registry. Ministry of Health & Welfare. 2006. Retrieved October 20, 2006. from http://www.mw.go.kr/user.tdf.
10. Survey report on wage structure. Ministry of Labor. 2006. Retrieved October 20, 2006. from http://www.molab.go.kr.
11. Oh K, Kim MJ, Kim KS, Park JW, Sung MS, Oh EG, et al. Educational issues and strategies to improve APN education. Journal of Korean Academy of Nursing. 2007. 37:801–809.

12. Park HJ, Shin HS. The effects of mouth care with sterile normal saline on chemotherapy-induced stomatitis. Journal of Korean Academy of Nursing. 1995. 25:5–16.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download