Abstract
Background
Type 1 diabetes mellitus is an autoimmune disease resulting in destruction of the pancreatic beta cells. Insulin gene therapy for these patients has been vigorously researched. The strategy for achieving glucose-dependent insulin secretion in gene therapy relies on glucose-responsive transcription of insulin mRNA and the constitutive secretory pathway of target non-beta cells. We observed that genetically engineered K-cells using Epstein-Barr virus (EBV)-based episomal vector can produce glucose-regulated insulin production.
Methods
Green fluorescent protein (GFP) or rat-preproinsulin (PPI) expression cassette transcriptionally controlled by the promoter of glucose dependent insulinotropic peptide (GIPP) is fused to pCEP4 containing the origin of replication (oriP) and Epstein-Barr virus nuclear antigen 1 (EBNA-1). CMV promoter was replaced by subcloning the GIPP into pCEP4 to generate pGIPP/CEP4. Two recombinant EBV-based episomal vectors, pGIPP/GFP/CEP4 and pGIPP/PPI/CEP4, were constructed. pGIPP/GFP/CEP4 and pGIPP/PPI/CEP4 containing K-cell specific GIPP were co-transfected into STC-1. K-cell was isolated from the clonal expansion of the fluorescent cells selected by hygromycin treatment in STC-1, and were analyzed for the expression of glucokinase (GK) or transcription factors involved in pancreas development. K-cells concurrently transfected with pGIPP/PPI/CEP4 and pGIPP/GFP/CEP4 were analyzed for the transcripts of PPI by RT-PCR, and for the glucose dependent insulin expression by immunocytochemistry or insulin assay using ultra-sensitive rat-specific insulin ELISA kit.
Result
STC-1 was stably-transfected with pGIPP/GFP/CEP4 along with pGIPP/PPI/CEP4. Genetically selected fluorescent K-cells expressed GK and transcription factors involved in pancreas development. And K-cells transfected with pGIPP/PPI/CEP4 contained detectable levels of PPI transcripts and showed glucose-dependent immunoreactive insulin secretion.
Conclusion
We identified genetically engineered K-cells which exert a glucose-dependent insulin expression using EBV-based episomal vector. The similarities between K-cells and pancreatic beta cells support that K-cells may make effective and ideal targeting cells for insulin gene therapy or alternative cell therapy.
Figures and Tables
Fig. 1
Schematic representation of the cloning steps employed to produce the pGIPP/GFP/CEP4 vector.
(A) EBV-based plasmid (pCEP4) vector containing the EBV nuclear antigen 1 (EBNA-1) and the origin of replication (oriP). CMV promoter excised as BglII/NotI was replaced by rat GIP promoter (GIPP) which was digested with BamHI and NotI from pGIPP/CR2.1. Then, GFP fragment excised with XhoI from pGFP/EGFP-C2 was fused to the XhoI restriction site of pGIPP/CEP4 to generate pGIPP/GFP/CEP4. (B) Linear structure of the pGIPP/GFP/CEP4 showing the BglII/BamHI cloning site.
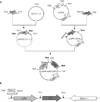
Fig. 2
Schematic representation for the construction of insulin gene expression cassettes.
(A) Rat preproinsulin (PPI) cDNA amplified by the primer set including BamHI and XhoI site was incorporated into the pBluescript or pGEX vector. From the pPPI/Bluescript vector, PPI was excised as a NotI/XhoI and purified by agarose gel electrophoresis. Then PPI fragment was fused to the NotI/XhoI restriction site of the pGIPP/CEP4 vector to generate pGIPP/PPI/CEP4. (B), (C) Linear structure of the pGIPP/PPI/CEP4 and pPPI/GEX vector, respectively.
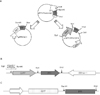
Fig. 3
Confirmation of cloned vector.
A, PCR analysis of rat GIPP from pGIPP/CR2.1 vector; B, PCR analysis of rat PPI from pPPI/CR2.1 vector. GIPP or PPI gene is shown well incorporated into pCR2.1 TOPO Vector; C, pGIPP/PPI/CEP4 digested with NotI and SalI; D, Cloned expression vector of pGIPP/GFP/CEP4 digested with XhoI and SalI respectively. GIPP or PPI, and GIPP or GFP were digested well, and the nucleotide sequences were confirmed by sequence analysis (data not shown).
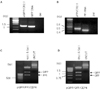
Fig. 4
Expression of insulin from pPPI/GEX vector in Escherichia coli.
Western blot analysis of insulin from Escherichia coli expressing GST only (lanes 1, 2), negative control (lanes 3, 4), GST-tagged insulin (lanes 5, 6), and bead binding GST-tagged insulin (lane 7) were separated on 15% (w/v) SDS PAGE. Arrows indicate the induced GST only (lane 1-2) and GST-tagged insulin (lane 5-7). GST or rat-insulin antibody recognized a GST or GST-tagged insulin. A, lysates prior to IPTG induction; B, lysates after IPTG induction.

Fig. 5
Isolation of K-cell from STC-1 cell line.
A, Schematic diagram of the isolation steps employed to separation from the K-cells; B, Fluorescence microscopic findings of the isolated K-cells from STC-1. Phase-contrast (I), fluorescence (II), and merge (III) micrographs of the STC-1 cells co-transfected with pGIPP/GFP/CEP4 and pGIPP/PPI/CEP4. Transfected cells with pGIPP/GFP/CEP4 emit green fluorescence (1), transfected cells formed a clone (2), and continuously subcultured cells (K-cells) emit green fluorescence (3).

Fig. 6
Western blot analysis and RT-PCR analysis in K-cell.
A, Western blot analysis of glucokinase (GK) expression in K-cell and STC-1. Bovine aortic endothelial cell (BAEC) was used as a negative control and MIN6 was used as a positive control. More GK is expressed in K-cells than in STC-1, but not in BAEC; B, RT-PCR analysis of transcription factors related to pancreas development and β-cell functions represent K-cell is comparable to mouse islet cells. Nkx 6.1 and ngn3 were not expressed in K-cells. Pax4 was in K-cell but not in mouse islet cells. Lane MW: 2 ug 1-kb ladder; lane 1: k-cells; lane 2: mouse islet cells.

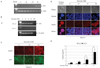
Fig. 7
Insulin secretion analysis from K-cells.
A, RT-PCR for the selection of rat-preproinsulin mRNA in four different clones. K-cells transfected with pGIPP/PPI/CEP4 (in clone 4 only) expressed preproinsulin mRNA; B, RT-PCR analysis for preproinsulin mRNA from clone 4 cells in response to glucose. Samples were prepared either in the presence (+) or absence (-) of reverse transcriptase (RT); C, D, Immunocytochemical staining for insulin expression from the co-transfected with pGIPP/GFP/CEP4 and pGIPP/PPI/CEP4. Insulin expression showed glucose dependent manner. E, Effects of glucose on the insulin secretion from K-cells stably transfected with the pGIPP/PPI/CEP4 (clone 4 cells) compared with the pGIPP/GFP/CEP4 (clone 3 cells). Results are means ± SD from three independent triplicate experiments in each group.
*P < 0.01 vs clone 3.
References
1. Yoon JW, Jun HS. Recent advances in insulin gene therapy for type 1 diabetes. Trends Mol Med. 2002. 8:62–68.
2. Morral N. Gene therapy for type 1 diabetes. New approaches. Minerva Med. 2004. 95:93–104.
3. Steiner DF, Rouille Y, Gong Q, Martin S, Carroll R, Chan SJ. The role of prohormone convertases in insulin biosynthesis: evidence for inherited defects in their action in man and experimental animals. Diabetes Metab. 1996. 22:94–104.
4. Tang SC, Sambanis A. Development of genetically engineered human intestinal cells for regulated insulin secretion using rAAV-mediated gene transfer. Biochem Biophys Res Commun. 2003. 303:645–652.
5. Nett PC, Sollinger HW, Alam T. Hepatic insulin gene therapy in insulin-dependent diabetes mellitus. Am J Transplant. 2003. 3:1197–1203.
6. Stewart C, Taylor NA, Green IC, Docherty K, Bailey CJ. Insulin-releasing pituitary cells as a model for somatic cell gene therapy in diabetes mellitus. J Endocrinol. 1994. 142:339–343.
7. Olson DE, Paveglio SA, Huey PU, Porter MH, Thule PM. Glucose-responsive hepatic insulin gene therapy of spontaneously diabetic BB/Wor rats. Hum Gene Ther. 2003. 14:1401–1413.
8. Yoon JW, Jun HS. Recent advances in insulin gene therapy for type 1 diabetes. Trends in Molecular Medicine. 2002. 8:62–68.
9. Thule PM, Campbell AG, Kleinhenz DJ, Olson DE, Boutwell JJ, Sutliff RL, Hart CM. Hepatic insulin gene therapy prevents deterioration of vascular function and improves adipocytokine profile in STZ-diabetic rats. Am J Physiol Endocrinol Metab. 2006. 290:E114–E122.
10. Stewart C, Taylor NA, Docherty K, Bailey CJ. Insulin delivery by somatic cell gene therapy. J Mol Endocrinol. 1993. 11:335–341.
11. Bailey CJ, Davies EL, Docherty K. Prospects for insulin delivery by ex-vivo somatic cell gene therapy. J Mol Med. 1999. 77:244–249.
12. Cheung AT, Dayanandan B, Lewis JT, Korbutt GS, Rajotte RV, Bryer-Ash M, Boylan MO, Wolfe MM, Kieffer TJ. Glucose-dependent insulin release from genetically engineered K cells. Science. 2000. 290:1959–1962.
13. Ramshur EB, Rull TR, Wice BM. Novel insulin/GIP co-producing cell lines provide unexpected insights into Gut K-cell function in vivo. J Cell Physiol. 2002. 192:339–350.
14. Bailey CJ, Flatt PR, Kwasowski P, Powell CJ, Marks V. Immunoreactive gastric inhibitory polypeptide and K cell hyperplasia in obese hyperglycaemic (ob/ob) mice fed high fat and high carbohydrate cafeteria diets. Acta Endocrinol(Copenh). 1986. 112:224–229.
15. Corbett JA. K cells: a novel target for insulin gene therapy for the prevention of diabetes. Trends Endocrinol Metab. 2001. 12:140–142.
16. Deacon CF. What do we know about the secretion and degradation of incretin hormones? Regul Pept. 2005. 128:117–124.
17. Irwin N, Gault VA, Green BD, Greer B, McCluskey JT, Harriott P, O'Harte FP, Flatt PR. Effects of short-term chemical ablation of the GIP receptor on insulin secretion, islet morphology and glucose homeostasis in mice. Biol Chem. 2004. 385:845–852.
18. Gremlich S, Porret A, Hani EH, Cherif D, Vionnet N, Froquel P, Thorens B. Cloning, functional expression, and chromosomal localization of the human pancreatic islet glucose-dependent insulinotropic polypeptide receptor. Diabetes. 1995. 44:1202–1208.
19. Buchan AM, Polak JM, Capella C, Solcia E, Pearse AG. Electronimmunocytochemical evidence for the K cell localization of gastric inhibitory polypeptide (GIP) in man. Histochemistry. 1978. 56:37–44.
20. Buffa R, Polak JM, Pearse AG, Solcia E, Grimelius L, Capella C. Identification of the intestinal cell storing gastric inhibitory peptide. Histochemistry. 1975. 43:249–255.
21. Min KA, Lee SK, Kim CK. Improved gene expression pattern using Epstein-Barr virus (EBV)-based plasmid and cationic emulsion. Biomaterials. 2005. 26:1063–1070.
22. Min KA, Oh ST, Yoon KH, Kim CK, Lee SK. Prolonged gene expression in primary porcine pancreatic cells using an Epstein-Barr virus-based episomal vector. Biochem Biophys Res Commun. 2003. 305:108–115.
23. Kim YD, Park KG, Morishita R, Kaneda Y, Kim SY, Song DK, Kim HS, Nam CW, Lee HC, Lee KU, Park JY, Kim BW, Kim JG, Lee IK. Liver-directed gene therapy of diabetic rats using an HVJ-E vector containing EBV plasmids expressing insulin and GLUT 2 transporter. Gene Ther. 2006. 13:216–224.
24. Egea JC, Hirtz C, Gross R, Lajoix AD, Traskawka E, Ribes G, de Periere DD. Preproinsulin I and II mRNA expression in adult rat submandibular glands. Eur J Oral Sci. 2000. 108:292–296.
25. Minami K, Okuno M, Miyawaki K, Okumachi A, Ishizaki K, Oyama K, Kawaquchi M, Ishizuka N, Iwanaga T, Seino S. Lineage tracing and characterization of insulin-secreting cells generated from adult pancreatic acinar cells. Proc Natl Acad Sci U S A. 2005. 102:15116–15121.
26. Kajihara M, Sone H, Amemiya M, Katoh Y, Isoqai M, Shimano H, Yamada N, Takahashi S. Mouse MafA, homologue of zebrafish somite Maf 1, contributes to the specific transcriptional activity through the insulin promoter. Biochem Biophys Res Commun. 2003. 312:831–842.
27. Boylan MO, Jepeal LI, Jarboe LA, Wolfe MM. Cell-specific expression of the glucose-dependent insulinotropic polypeptide gene in a mouse neuroendocrine tumor cell line. J Biol Chem. 1997. 272:17438–17443.
28. Lee HC, Kim SJ, Kim KS, Shin HC, Yoon JW. Remission in models of type 1 diabetes by gene therapy using a single-chain insulin analogue. Nature. 2000. 408:483–488.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download