Abstract
Background
Diabetic neuropathy is associated with risk factors for macrovascular diseases and other microvascular complications. Alpha-lipoic acid (ALA) administration has been reported to improve metabolic abnormalities and ameliorate peripheral polyneuropathy in diabetic patients. In addition, ALA improves endoneurial nutritive neural blood flow and nerve conduction velocity in diabetic rats. But it is not clear whether ALA has a preservation effect on microvasculature in addition to the effect on intraepidermal nerve fibers (IENFs). We investigated the effect of ALA on intraepidermal nerve fiber density (numbers/mm) and cutaneous capillary length in streptozotocin-induced diabetic rats.
Methods
The rats were randomly divided into 3 groups: diabetes without diet control, diabetes with diet control, and diabetes with ALA treatment. Diabetes was induced by a single intraperitoneal injection of streptozotocin (60 mg/kg) and the effect of ALA treatment was assessed by IENF immunostained with protein gene product 9.5 and by quantification of total cutaneous capillary length with mouse anti-rat reca-1 immunostaining.
Results
The value of IENF density significantly increased in ALA treatment group compared with other groups (P < 0.05). Quantification of microvascularity was also significantly increased in ALA treatment group compared with other groups (P < 0.05).
Conclusion
The results of this study suggest that ALA administration in diabetic rats may be beneficial in the prevention of peripheral neuropathy associated with improvement of microvascularity. And the symptomatic amelioration after ALA treatment may be attributed to this morphological improvement.
Figures and Tables
Fig. 1
A. Body weight changes of animals according to the management in the experimental period. B. Fasting blood glucose levels of animals according to the management in the experimental period. N = 5 in each group. ALA, alpha lipoic acid. *P < 0.05 vs DM without diet control.

Fig. 2
A. Blood glucose level after oral glucose challenge according to the management in the experimental period. B. Blood glucose level after intraperitoneal insulin challenge according to the management in the experimental period. N = 5 in each group. ALA, alpha-lipoic Acid; ITT, insulin tolerance test. *P < 0.05 vs. DM without diet control. †P < 0.05 vs. DM with diet control.

Fig. 3
The level of insulin secretion in the isolated beta cell in response to 20 mM glucose medium. All values are presented as mean ± SD. N = 5 in each group. ALA, alpha-lipoic Acid. *P < 0.05 vs. DM without diet control.

Fig. 4
Intraepidermal nerve fiber density according to the experimental group. N= 5 in each group. ALA, alpha lipoic acid. *P < 0.05 vs. DM without diet control. †P < 0.05 vs. DM with diet control.

Fig. 5
Morphological changes of dermal and epidermal nerve fibers according to the management. A. DM without diet control group. B. DM with diet control group. C. DM with ALA treatment group. ↔ Arrow indicates epidermis. → Arrow indicates intraepidermal nerve fiber (×100).
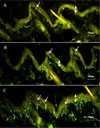
Fig. 6
Total capillary length according to the experimental group. N = 5 in each group. ALA, alpha lipoic acid. *P < 0.05 vs. DM without diet control; †P < 0.05 vs. DM with diet control.

Fig. 7
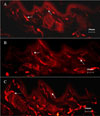
Morphological changes of dermal and epidermal blood vessels according to the management in the experimental animals shown by endothelial cell antibody RECA-1 immunostaining. A. DM without diet control group. B. DM with diet control group. C. DM with ALA treatment group. ALA, alpha lipoic acid. → Arrow indicates cutaneous capillary (×100).
References
1. The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993. 329:977–986.
2. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulfonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998. 352:837–853.
3. Parry GJ. Management of diabetic neuropathy. Am J Med. 1999. 107:27s–33s.
4. Boulton AJ, Malik RA, Arezzo JC, Sosenko JM. Diabetic Somatic Neuropathies. Diabetes Care. 2004. 27:1458–1486.
5. Ziegler D, Gries FA. Alpha-lipoic acid in the treatment of diabetic peripheral and cardiac autonomic neuropathy. Diabetes. 1997. 46:Suppl 2. S62–S66.
6. Yan SD, Schmidt AM, Anderson GM, Zhang J, Brett J, Zou YS, Pinsky D, Stern D. Enhanced cellular oxidant stress by the interaction of advanced glycation end products with their receptors/binding proteins. J Biol Chem. 1994. 269:9889–9897.
7. Son SM, Whalin MK, Harrison DG, Taylor WR, Griendling KK. Oxidative stress and diabetic vascular complications. Curr Diab Rep. 2004. 4:247–252.
8. Kerr S, Brosnan MJ, McIntyre M, Reid JL, Dominiczak AF, Hamilton CA. Superoxide anion production is increased in a model of genetic hypertension: role of the endothelium. Hypertension. 1999. 33:1353–1358.
9. Miller FJ Jr, Gutterman DD, Rios CD, Heistad DD, Davidson BL. Superoxide production in vascular smooth muscle contributes to oxidative stress and impaired relaxation in atherosclerosis. Circ Res. 1998. 82:1298–1305.
10. Inoguchi T, Li P, Umeda F, Yu HY, Kakimoto M, Imamura M, Aoki T, Etoh T, Hashimoto T, Naruse M, Sano H, Utsumi H, Nawata H. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes. 2000. 49:1939–1945.
11. Zou MH, Shi C, Cohen RA. High glucose via peroxynitrite causes tyrosine nitration and inactivation of prostacyclin synthase that is associated with thromboxane/prostaglandin H(2) receptor-mediated apoptosis and adhesion molecule expression in cultured human aortic endothelial cells. Diabetes. 2002. 51:198–203.
12. Ziegler D, Nowak H, Kempler P, Vargha P, Low PA. Treatment of symptomatic diabetic polyneuropathy with the antioxidant alpha-lipoic acid: a meta-analysis. Diabet Med. 2004. 21:114–121.
13. Kim HR, Rho HW, Park BH, Park JW, Kim JS, Kim UH, Chung MY. Role of Ca2+ in alloxan-induced pancreatic beta-cell damage. Biochim Biophys Acta. 1994. 1227:87–91.
14. Park BH, Rho HW, Park JW, Cho CG, Kim JS, Chung HT, Kim HR. Protective mechanism of glucose against alloxan-induced pancreatic beta-cell damage. Biochem Biophys Res Commun. 1995. 5:1–6.
15. Wild R, Ramakrishnan S, Sedgewick J, Griffioen AW. Quantitative assessment of angiogenesis and tumor vessel architecture by computer-assisted digital image analysis: effects of VEGF-toxin conjugate on tumor microvessel density. Microvasc Res. 2000. 59:368–376.
16. Low PA, Nickander KK. Oxygen free radical effects in sciatic nerve in experimental diabetes. Diabetes. 1991. 40:873–877.
17. Nickander KK, Schmelzer JD, Rohwer DA, Low PA. Effect of alpha-tocopherol deficiency on indices of oxidative stress in normal and diabetic peripheral nerve. J Neurol Sci. 1994. 126:6–14.
18. Cameron NE, Cotter MA. The relationship of vascular changes to metabolic factors in diabetes mellitus and their role in the development of peripheral nerve complications. Diabetes Metab Rev. 1994. 10:189–224.
19. Schmidt AM, Hori O, Brett J, Yan SD, Wautler JL, Stern D. Cellular receptors for advanced glycation end products: implications for induction of oxidant stress and cellular dysfunction in the pathogenesis of vascular lesions. Arterioscle Thromb Vasc Biol. 1994. 14:1521–1528.
20. Frustaci A, Kajstura J, Chimenti C, Jakoniuk I, Leri A, Maseri A, Nadal-Ginard B, Anversa P. Myocardial cell death in human diabetes. Circ Res. 2000. 87:1123–1132.
21. Hunt JV, Dean RT, Wolff SP. Hydroxy radical production and autoxidative glycosylation:glucose autoxidation as the cause of protein damage in the experimental glycation model of diabetes mellitus and aging. Biochem J. 1988. 256:205–212.
22. Matsubara T, Ziff M. Increased superoxide anion release from human endothelial cells in response to cytokines. J Immunol. 1986. 137:3295–3298.
23. Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Cir Res. 1994. 74:1141–1148.
24. Pagano PJ, Chanock SJ, Siwik DA, Colucci WS, Clark JK. Angiotensin II induces p67phox mRNA expression and NADPH oxidase superoxide generation in rabbit aortic adventitial fibroblasts. Hypertension. 1998. 32:331–337.
25. Anand P, Terenghi G, Warner G, Kopelman P, Wiliams-Chestnut RE, Sinicropi DV. The role of endogenous nerve growth factor in human diabetic neuropathy. Nature Medicine. 1996. 2:703–707.
26. Greene DA, Stevens MJ, Obrosova I, Feldman EL. Glucose-induced oxidative stress and programmed cell death in diabetic neuropathy. Eur J Pharmacol. 1999. 375:217–223.
27. Sima AA. New insights into the metabolic and molecular basis for diabetic neuropathy. Cell Mol Life Sci. 2003. 60:2445–2464.
28. Wang L, Hilliges M, Jernberg T. Protein gene product 9.5-immunoreactive nerve fibres and cells in human skin. Cell Tissue Res. 1990. 261:25–33.
29. Masson EA, Boulton AJ. Aldose reductase inhibitors in the treatment of diabetic neuropathy. A review of the rationale and clinical evidence. Drugs. 1990. 39:190–202.
30. Ziegler D, Mayer P, Rathmann W, Gries FA. One-year treatment with the aldose reductase inhibitor, ponalrestat, in diabetic neuropathy. Diabetes Res Clin Pract. 1991. 14:63–73.
31. Jamal GA. The use of gamma linolenic acid in the prevention and treatment of diabetic neuropathy. Diabet Med. 1994. 11:145–149.
32. Veresiu IA. Treatment of diabetic polyneuropathy with alpha-lipoic acid is evidence based. Rom J Intern Med. 2004. 42:293–299.
33. Ziegler D, Nowak H, Kempler P, Vargha P, Low PA. Treatment of symptomatic diabetic polyneuropathy with the antioxidant alpha-lipoic acid: a meta-analysis. Diabet Med. 2004. 21:114–121.
34. Tankova T, Cherninkova S, Koev D. Treatment for diabetic mononeuropathy with alpha-lipoic acid. Int J Clin Pract. 2005. 59:645–650.
35. Yoshida K, Hirokawa J, Tagami S, Kawakami Y, Urata Y, Kondo T. Weakened cellular scavenging activity against oxidative stress in diabetes mellitus: regulation of glutathione synthesis and efflux. Diabetologia. 1995. 38:201–210.
36. Tominaga M, Kimura M, Sugiyama K, Abe T, Igarashi K, Igarashi M, Eguchi H, Sekikawa A, Ogawa A, Manaka H. Effects of seishin-renshi-in and Gymnema sylvestre on insulin resistance in streptozotocin-induced diabetic rats. Diabetes Res Clin Pract. 1995. 29:11–17.
37. Kim YW, Kim JY, Lee SK. Effects of phlorizin and acipimox on insulin resistance in STZ-diabetic rats. J Korean Med Sci. 1995. 10:24–30.
38. Sanchez-Arias JA, Sanchez-Gutierrez JC, Guadano A, Alvarez JF, Samper B, Mato JM, Feliu JE. Impairment of glycosyl-phosphatidylinositol-dependent insulin signaling system in isolated rat hepatocytes by streptozotocin-induced diabetes. Endocrinology. 1992. 131:1727–1733.
39. Ahmad F, Goldstein BJ. Alterations in specific protein-tyrosine phosphatases accompany insulin resistance of streptozotocin diabetes. Am J Physiol. 1995. 268:932–940.
40. Bonini JA, Colca J, Hofmann C. Altered expression of insulin signaling components in streptozotocin-treated rats. Biochem Biophys Res Commun. 1995. 212:933–938.
41. Targonsky ED, Dai F, Koshkin V, Karaman GT, Gyulkhandanyan AV, Zhang Y, Chan CB, Wheeler MB. alpha-lipoic acid regulates AMP-activated protein kinase and inhibits insulin secretion from beta cells. Diabetologia. 2006. 49:1587–1598.
42. Kim MS, Park JY, Namkoong C, Jang PG, Ryu JW, Song HS, Yun JY, Namgoong IS, Ha J, Park IS, Lee IK, Viollet B, Youn JH, Lee HK, Lee KU. Anti-obesity effects of alpha-lipoic acid mediated by suppression of hypothalamic AMP-activated protein kinase. Nat Med. 2004. 10:727–733.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download