Abstract
Background
Delayed wound healings in diabetic patients are related with the impairment of the expressions of various growth factors. Treatments using growth factors have been attempted on diabetic foot ulcer. VEGF (vascular endothelial growth factor) accelerates neo-angiogenesis on the early phase of the wound healing and exerts chemo-attractive effect for the other growth factors and cytokines. Non-viral gene transfer strategies are attractive tool for the gene therapy due to the safety and the versatility, but the low efficiency has been the serious problem.
Methods
We performed the VEGF gene therapy using reconstructed minicircle MINI-pβVEGF DNA with a polymeric carrier, polyethylenimine (PEI, 25 kDa) in HEK293, CHO, and NIH3T3 cell lines, and compared its efficiency with the conventional VEGF plasmid pβVEGF.
Figures and Tables
 | Fig. 1Production of minicircle pβVEGF. A, L-(+)-arabinose (Sigma) was added on E.coli DH5a transformed with pBAD-pieC31-βVEGF plamid and the additional incubation for the integration of DNAs at 30℃ by times; B, The minicircle pβVEGF was separated by linearized the remnant bacterial backbone using EcoRV restriction enzyme. |
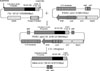 | Fig. 2Reconstruction of the minicircle pβVEGF. The expression cassette of pβVEGF comprised with chicken beta actin promoter, the VEGF165 cDNA and d SV40 poly adenylation signal sequence was excised with Bgl II and Cla I and ligated bluntly between attB and attP in plasmid pBAD-pieC31. The minicircle pβVEGF was produced by C31 integrase recombinating the att structure. |
 | Fig. 3Luciferase activity assay in HEK293 cell line. Luciferase activities were increased on cells transfected with DNA/BPEI polyplexes than naked plasmids (P < 0.05). And it was increased more one hundred times on minicircle pβVEGF than conventional pβVEGF on groups using BEPI (branched polyethylenimine), cataionic gene carrier. |
 | Fig. 4VEGF protein level by ELISA in HEK293, NIH3T3, and CHO cell line. VEGF proteins were more expressed on groups of BPEI complexes than naked plasmids in all cell lines (P < 0.05). VEGF expressions were more increased about 2.5, 2 and 1.25 times on minicircle pβVEGF group than conventional pβVEGF using BEPI in HEK293(A), NIH3T3(B), and CHO(C) cell line. |
References
1. Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004. 27:1047–1053.
2. Boulton AJ, Vileikyte L, Ragnarson-Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet. 2005. 366:1719–1724.
4. Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005. 366:1736–1743.
5. Bennett SP, Griffiths GD, Schor AM, Leese GP, Schor SL. Growth factors in the treatment of diabetic foot ulcers. Br J Surg. 2003. 90:133–146.
6. Harding KG, Morris HL, Patel GK. Science, medicine and the future: healing chronic wounds. BMJ. 2002. 324:160–163.
7. Pollard H, Remy JS, Loussouam G, Demolombe S, Behr JP, Escande D. Polyethylenimine but not cationic lipids promotes transgene delivery to the nucleus in mammalian cells. J Biol Chem. 1998. 273:7507–7511.
8. Sonawane ND, Szoka FC, Verkman AS. Chloride accumulation and swelling in endosomes enhances DNA transfer by polyamine-DNA polyplexes. J Biol Chem. 2003. 278:44826–44831.
9. Darquet AM, Rangara R, Kreiss P, Schwartz B, Naimi S, Delaere P, Crouzet J, Scherman D. Minicircle: an improved DNA molecule for in vitro and in vivo gene transfer. Gene Ther. 1999. 6:209–218.
10. Bigger BW, Tolmachov O, Collombet JM, Fragkos M, Palaszewski I, Coutelle C. An ARAC-controlled bacterial CRE expression system to produce DNA minicircle vectors for nuclear and mitochondrial gene therapy. J Biol Chem. 2001. 276:23018–23027.
11. Chen ZY, He CY, Ehrhardt A, Kay MA. Minicircle DNA vectors devoid of bacterial DNA result in persistent and high-level transgene expression in vivo. Mol Ther. 2003. 8:495–500.
12. Brem H, Tomic-Canic M. Cellular and molecular basis of wound healing in diabetes. J Clin Invest. 2007. 117:1219–1222.
13. Iruela-Arispe ML, Dvorak HF. Angiogenesis: a dynamic balance of stimulators and inhibitors. Thromb Haemost. 1997. 78:672–677.
14. Kirchner LM, Meerbaum SO, Gruber BS, Knoll AK, Bulgrin J, Taylor RAJ, Schmidt SP. Effects of vascular endothelial growth factor on wound closure rates in the genetically diabetic mouse model. Wound Repair Regen. 2003. 11:127–131.
15. Pandya NM, Dhalla NS, Santani DD. Angiogenesis-a new target for future therapy. Vascul Pharmacol. 2006. 44:265–274.
16. Frank S, Hbuner G, Breier G, Longaker M, Greenhalgh DG, Werner S. Regulation of vascular endothelial growth factor expression in cultured keratinocytes. J Biol Chem. 1995. 270:12607–12613.
17. Boussif O, Lezoualc'h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, Behr JP. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci USA. 1995. 92:7297–7301.
18. Thomas M, Klibanov AM. Enhancing polyethylenimine's delivery of plasmid DNA into mammalian cells. Proc Natl Acad Sci USA. 2002. 99:14640–14645.
19. Furgeson DY, Chan WS, Yockman JW, Kim SW. Modified linear polyethylenimine-cholesterol conjugates for DNA complexation. Bioconjug Chem. 2003. 14:840–847.
20. Nettelbeck DM, Jerome V, Muller R. A strategy for enhancing the transcriptional activity of weak cell type-specific promoters. Gene Ther. 1998. 5:1656–1664.
21. Natesan S, Molinari E, Rivera VM, Rickles RJ, Gilman M. A general strategy to enhance the potency of chimeric transcriptional activators. Proc Natl Acad Sci USA. 1999. 96:13898–13903.
22. Darquet AM, Cameron B, Wils P, Scherman D, Crouzet J. A new DNA vehicle for nonviral gene delivery: supercoiled minicircle. Gene Ther. 1997. 4:1341–1349.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download