Abstract
Background
Oxidative stress is induced under diabetic conditions and causes various forms of tissue damages in the patients with diabetes. Recently, pancreatic beta cells are regarded as a putative target of oxidative stress-induced tissue damage, and this seems to explain in part the progressive deterioration of beta cell function in type 2 diabetes. The aim of this study was to examine the potential of Quercetin (QE) to protect INS-1 cells from the H2O2-induced oxidative stress and the effects of QE on the glucose-stimulated insulin secretion in INS-1 cells.
Methods
To study the cell viability, cells were incubated with H2O2 and/or QE at the various concentrations. To confirm the protective effect by QE in response to H2O2, the levels of antioxidant enzymes were assessed by RT-PCR and Western blot, and glutathione peroxidase activities were quantified by spectrophotometrical method. Glucose-stimulated insulin secretion (GSIS) was measured by ELISA.
Results
Cell incubations were performed with 80 µM of H2O2 for 5 hours to induce 40 - 50% of cell death. QE gradually showed protective effect (IC50 = 50 µM) in dose-dependent manner. Superoxide dismutase (SOD) mRNA level in H2O2 + QE group was increased as compared to H2O2 group, but catalase did not changed. And the QE recruited glutathione peroxidase activity against H2O2-induced oxidative injuries in INS-1 cells.
Figures and Tables
 | Fig. 1H2O2-induced cytotoxicity in INS-1 cells. INS-1 cells exposed to various concentrations of H2O2(0, 10, 20, 50, 80 and 100 µM) for 5 hours, and then, cell viability was measured by MTT method. |
 | Fig. 2Effect of quercetin on INS-1 cells. INS-1 cells were treated with 80 µM of H2O2(IC50) and various concentration of quercetin (0, 10, 25, 50 and 100 µM) for 5 hours, and then, cell viability was measured with MTT method. |
 | Fig. 3The effects of quercetin (QE) on H2O2-induced cell damage in INS-1 cells. Morphology of INS-1 cells of control (A) and treated with 80 µM H2O2 (B) or 80 µM H2O2 + 50 µM QE (C) for 5 hours was observed by optical microscope (×200). |
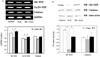 | Fig. 4The effect of quercetin on SOD and catalase expression in INS-1 cells. A, SOD and catalase mRNA expression in INS-1 cells of control and treated with H2O2 and/or Quercetin for 5 hours was evaluated by RT-PCR; B, Expression of SOD and catalase in INS-1 cells of all groups was detected by western blotting. C, control; QE, quercetin; WB, western blotting. *P < 0.05. |
 | Fig. 5The effect of quercetin (QE) on GPx activity. The GPx activity in contol, H2O2 and H2O2 + QE group was measured by Total Glutathione Quantification Kit and determined the change in absorbance per minute. C, control; GPx, Glutathione peroxidase; QE, quercetin. |
References
1. Kumar V, Fausto N, Abbas A. Robbins & Cotran Pathologic Basis of Disease: free radical induced cell injury. 2004. 7th ed. Philadelphia: W.B. Saunders;16–18.
2. Tiwari AK. Antioxidants: new-generation therapeutic base for treatment of polygenic disorders. Curr Sci. 2004. 86:1092–1102.
3. Cui K, Luo XL, Xu KY, Ven Murthy MR. Role of oxidative stress in neurodegeneration: recent developments in assay methods for oxidative stress and nutraceutical antioxidants. Prog Neuropsychopharmacol Biol Psychiatry. 2004. 28:771–799.
4. Ceriello A, Motz E. Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease? The common soil hypothesis revisited. Arterioscler Thromb Vasc Biol. 2004. 24:816–823.
5. Klaunig JE, Kamendulis LM. The role of oxidative stress in carcinogenesis. Annu Rev Pharmacol Toxicol. 2004. 44:239–267.
6. Ballinger SW. Mitochondrial dysfunction in cardiovascular disease. Free Radic Biol Med. 2005. 38:1278–1295.
7. Gibson GE, Huang HM. Oxidative stress in Alzheimer's disease. Neurobiol Aging. 2005. 26:575–578.
8. Grankvist K, Marklund SL, Taljedal IB. CuZn-superoxide dismutase, Mn-superoxide dismutase, catalase and glutathione peroxidase in pancreatic islets and other tissues in the mouse. Biochem J. 1981. 199:393–398.
9. Hanasaki Y, Ogawa S, Fukui S. The correlation between active oxygen scavenging and antioxidative effects of flavonoids. Free Radic Biol Med. 1994. 6:845–853.
10. Ihara Y, Toyokuni S, Uchida K, Odaka H, Tanaka T, Ikeda H, Hiai H, Seino Y, Yamada Y. Hyperglycemia causes oxidative stress in pancreatic beta-cells of GK rats a model of type 2 diabetes. Diabetes. 1999. 48:927–932.
11. Baynes JW. Role of oxidative stress in development of complication in diabetes. Diabetes. 1991. 40:405–412.
12. Szkudelski T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res. 2001. 50:536–546.
13. Kubisch HM, Wang J, Bray TM, Phillips JP. Targeted overexpression of Cu/Zn superoxide dismutase protects pancreatic b-cells against oxidative stress. Diabetes. 1997. 46:1563–1566.
14. Plumb W, Price KR, Williamson G. Antioxidant properties of flavonol glycosides from green beans. Redox Rep. 1999. 4:123–127.
15. Fiorani M, Sanctis R, Menghinello P, Cucchiarini L, Cellini B, Dacha M. Quercerin prevents glutathione depletion induced by dehydroascorbic acid in rabbit red blood cells. Free Radic Res. 2001. 34:639–648.
16. Baynes JW, Thorpe SR. The role of oxidative stress in diabetic complications. Curr Opin Endocrinol Diabetes Obes. 1996. 3:277–284.
17. Ceriello A. New insights on oxidative stress and diabetic complications may lead to a causal antioxidant therapy. Diabetes Care. 2003. 26:1589–1596.
18. Lawerence MC, Bhatt HS, Watterson JM, Easom RA. Regulation of insulin gene transcription by a Ca2+ responsive pathway involving calcineurin and nuclear factor of activated T cells. Mol Endocrinol. 2001. 15:1758–1767.
19. West IC. Radicals and oxidative stress in diabetes. Diabet Med. 2000. 17:171–180.
20. Robertson RP, Zhang HJ, Pyzdrowski KL, Walseth TF. Preservation of insulin mRNA levels and insulin secretion in HIT cells by avoidance of chronic exposure to high glucose concentration. J Clin Invest. 1992. 90:320–325.
21. Wolff SP, Dean RT. Glucose autoxidation and protein modification. The potential role of autoxidative glycosylation in diabetes. Biochem J. 1987. 245:243–250.
22. Hunt JV, Dean RT, Wolff SP. Hydroxyl radical production and autoxidative glycosylation, Glucose autoxidation as the cause of protein damage in the experimental glycation model of diabetes mellitus and ageing. Biochem J. 1988. 256:205–212.
23. Suarez-Pinzon WL, Strynadka K, Rabinovitch A. Destruction of rat pancreatic islet beta-cells by cytokines involves the production of cytotoxic aldehydes. Endocrinology. 1996. 137:5290–5296.
26. Robertson RP. Chronic oxidative stress as a central mechanism for glucose toxicity in pancreatic islet beta cells in diabetes. J Biol Chem. 2004. 279:42351–42354.
27. Grankvist K, Marklund SL, Taljedal IB. Superoxide dismutase is a prophylactic against alloxan diabetes. Nature. 1981. 294:158–160.
28. Benhamou PY, Moriscot C, Richard MJ, Beatrix O, Badet L, Pattou F, Kerr-Conte J, Chroboczek J, Lemarchand P, Halimi S. Adenovirus-mediated catalase gene transfer reduces oxidant stress in human, porcine and rat pancreatic islets. Diabetologia. 1998. 41:1093–1100.
29. Moriscot C, Pattou F, Kerr-Conte J, Richard MJ, Lemarchand P, Benhamou PY. Contribution of adenoviral-mediated superoxide dismutase gene transfer to the reduction in nitric oxide-induced cytotoxicity on human islets and INS-1 insulin-secreting cells. Diabetologia. 2000. 43:625–631.
30. Tanaka Y, Gleason CE, Trans PO, Harmon JS, Robertson RP. Prevention of glucose toxicity in HIT-T15 cells and Zucker diabetic fatty rats by antioxidants. Proc Natl Acad Sci U S A. 1999. 96:10857–10862.
31. Kaneto H, Kajimoto Y, Miyagawa J, Matsuoka T, Fujitani Y, Umayahara Y, Hanafusa T, Matsuzawa Y, Yamasaki Y, Hori M. Beneficial effects of antioxidants in diabetes: possible protection of pancreatic β-cells against glucose toxicity. Diabetes. 1999. 48:2398–2406.
32. Ihara Y, Yamada Y, Toyokuni S, Miyawaki K, Ban N, Adachi T, Kuroe A, Iwakura T, Kubota A, Hiai H, Seino Y. Antioxidant α-tocopherol ameliorates glycemic control of GK rats, a model of type 2 diabetes. FEBS Lett. 2000. 473:24–26.
33. Moskaug JO, Carlsen H, Myhrstad M, Blomhoff R. Molecular imaging of the biological effects of quercetin and quercetin-rich food. Mech Ageing Dev. 2004. 125:315–324.
34. William M, Amanda JS, Michael EJL, Peter G, Garry GD, Alan C. Effect of freezing and storage on the phenolics, ellagitannins, flavonoids, and antioxidant capacity of red raspberries. J Agric Food Chem. 1999. 50:5197–5201.
35. Hakkinen SH, Karenlampi SO, Heinonen IM, Mykkanen HM, Torronen AR. Content of the flavonols quercetin, myricetin, and kaempferol in 25 edible berries. J Agric Food Chem. 1999. 47:2274–2279.
36. Formica JV, Regelson W. Review of the biology of quercetin and related bioflavonoids. Food Chem Toxicol. 1995. 33:1061–1080.
37. Gadow AV, Joubert E, Hansmann CF. Comparison of the antioxidant activity of aspalathin with that of other plant phenols of rooibos tea(Aspalathus linearis), α-tocopherol, BHT, and BHA. J Agric Food Chem. 1997. 45:632–638.
38. Pietta PG. Flavonoids as antioxidants. J Nat Prod. 2000. 63:1035–1042.
39. Ferguson LR. Role of plant polyphenols in genomic stability. Mutat Res. 2001. 475:89–111.
40. Nagata H, Takekoshi S, Takagi T, Honma T, Watanabe K. Antioxidative action of flavonoids, quercetin and catechin, mediated by the activation of glutathione peroxidase. Tokai J Exp Clin Med. 1999. 24:1–11.
41. Chen TJ, Jeng JY, Lin CW, Wu CY, Chen YC. Quercetin inhibition of ROS-dependent and -independent apoptosis in rat glioma C6 cells. Toxicology. 2006. 223:113–126.
42. Scalbert A, Williamson G. Dietary intake and bioavailability of polyphenols. J Nutr. 2000. 130:2073S–2085S.
43. Manach C, Williamson G, Morand C, Scalbert A, Remesy C. Bioavailability and bioefficacy of polyphenols in humans. Review of 97 bioavailability studies. Am J Clin Nutr. 2005. 81:230S–242S.
44. Vessal M, Hemmati M, Vasei M. Antidiabetic effects of quercetin in streptozocin-induced diabetic rats. Comp Biochem Physiol C Toxicol Pharmacol. 2003. 135:357–364.
45. Coskun O, Kanter M, Korkmaz A, Oter S. Quercetin, a flavonoid antioxidant, prevents and protects streptozotocin-induced oxidative stress and β-cell damage in rat pancreas. Pharmacol Res. 2005. 51:117–123.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download