Abstract
Background
Hyperglycemia is a well-recognized pathogenic factor of long term complications in diabetes mellitus and hyperglycemia also generates reactive oxygen species (ROS) in β cells when ROS accumulate in excess for prolonged periods of time, they cause chronic oxidative stress and adverse effects. Unfortunately, the islet contacts low capacity of endogenous antioxidant effects. But, γ-glutamylcysteine synthetase (γ-GCS), the rate-limiting enzyme for glutathione synthesis, is well represented in islets.
Methods
This study is to evaluate the changes in the activity of γ-GCS, glutathione in β-cells exposed to high glucose, in pancreatic tissue of OLETF (Otsuka Long Evans Tokushima Fatty) and LETO (Long-Evans Tokushima Otsuka) rats, in leukocytes from patients with Korean type 2 DM (T2DM) and to disclose the effects of high blood glucose on this impairment in patients with T2DM. We divided our patients into 3 groups by HbA1c (controls: n = 20, well controls diabetes: n=24, poorly controlled diabetes: n = 36).
Results
We observed that decreased glutathione level, γ-GCS expression, glucose-stimulated (GSIS) and increased intracellular peroxide level in the INS-1 cells exposed to 30 mM glucose condition. Also decreased glutathione level at erythrocytes, γ-GCS expression at leukocytes and increased oxidized LDL, MDA (malondialdehyde) level at plasma from patients with T2DM compared to controls (esp, poorly controlled patients).
Figures and Tables
Fig. 1
The effects of high glucose on the intracellular peroxide level, PDX-1, MafA, insulin mRNA and glucose stimulated insulin secretion (GSIS) in the INS-1 cells. A, INS-1 cells incubated for 72 hours at 30 mM glucose increased levels of intracellular peroxides compared with the 5.6 mM glucose (P < 0.05). And, INS-1 cells incubated at 30 mM glucose decreased GSIS compared with the 5.6 mM glucose. *P < 0.05 vs. 5.6 mM glucose; B, INS-1 cells incubated for 72 hours at 30 mM glucose decreased PDX-1, MafA, insulin mRNA compared with the 5.6 mM glucose. *P < 0.05; C, INS-1 cells incubated for 72 hours at 30 mM glucose decreased the expression of γ-GCS mRNA and GSH level compared with the 5.6 mM glucose in INS-1 cell (P < 0.05). *P < 0.05 vs. 30 mM glucose. Data are mean ± SE from 3 separate experiments.
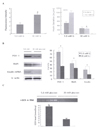
Fig. 2
GSH level at pancreatic tissues in OLETF (n = 5) and LETO rats (n = 5). GSH level at pancreatic tissues in OLETF rats was significantly decreased compared with LETO rats. *P < 0.05 vs. LETO rats.

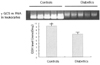
Fig. 3
Expression of γ-GCS mRNA in leukocytes and GSH level in erythrocytes from patients with T2DM. Expression of γ-GCS mRNA in leukocytes and GSH level in erythrocytes from patients with T2DM (n = 60) were significantly decreased compared with controls (n = 20). *P < 0.05 vs. Diabetics.
References
1. Robertson RP. Chronic oxidative stress as a central mechanism for glucose toxicity in pancreatic islet beta cells in diabetes. J Biol Chem. 2004. 279:42351–42354.
2. Rossetti L, Giaccari A, De Fronzo RA. Glucose toxicity. Diabetes Care. 1990. 13:610–630.
3. Robertson RP, Zhang HJ, Pyzdrowski KL, Walseth TF. Preservation of insulin mRNA levels and insulin secretion in HIT cells by avoidance of chronic exposure to high glucose concentrations. J Clin Invest. 1992. 90:320–325.
4. Olson LK, Redmon JB, Towle HC, Robertson RP. Chronic exposure of HIT cells to high glucose concentrations paradoxically decreases insulin gene transcription and alters binding of insulin gene regulatory protein. J Clin Invest. 1993. 92:514–519.
5. Robertson RP, Olson LK, Zhang HJ. Differentiating glucose toxicity from glucose desensitization: a new message from the insulin gene. Diabetes. 1994. 43:1085–1089.
6. Poitout V, Olson LK, Robertson RP. Chronic Exposure of βTC-6 Cells to Supraphysiologic Concentrations of Glucose Decreases Binding of the RIPE3b1 Insulin Gene Transcription Activator. J Clin Invest. 1996. 97:1041–1046.
7. Harmon JS, Stein R, Robertson RO. Oxidative stress-mediated, post-translational loss of MafA protein as a contributing mechanism to loss of insulin gene expression in glucotoxic beta cells. J Biol Chem. 2005. 280:11107–11113.
8. Tanaka Y, Tran PO, Harmon J, Robertson RP. A role for glutathione peroxidase in protecting pancreatic beta cells against oxidative stress in a model of glucose toxicity. Proc Natl Acad Sci USA. 2002. 99:12363–12368.
10. Griffith OW, Mulcahy RT. The enzymes of glutathione synthesis: gamma-glutamylcysteine synthetase. Adv Enzymol Relat Areas Mol Biol. 1999. 73:209–267.
11. Hayes JD, McLellan LI. Glutathione and glutathione-dependent enzymes represent a co-ordinately regulated defence against oxidative stress. Free Radic Res. 1999. 31:273–300.
12. Adams JD Jr, Lauterburg BH, Mitchell JR. Plasma glutathione and glutathione disulfide in the rat: regulation and response to oxidative stress. J Pharmacol Exp Ther. 1983. 227:749–754.
13. Murakami K, Kondo T, Ohtsuka Y, Fujiwara Y, Shimada M, Kawakami Y. Impairment of glutathione metabolism in erythrocytes from patients with diabetes mellitus. Metabolism. 1989. 38:753–758.
14. Sharma A, Kharb S, Chugh SN, Kakkar R, Singh GP. Evaluation of oxidative stress before and after control of glycemia and after vitamin E supplementation in diabetic patients. Metabolism. 2000. 49:160–162.
15. Yoshida K, Hirokawa J, Tagami S, Kawakami Y, Urata Y, Kondo T. Weakened cellular scavenging activity against oxidative stress in diabetes mellitus: regulation of glutathione synthesis and efflux. Diabetologia. 1995. 38:201–210.
16. Kawano K, Hirashima T, Mori S, Saitoh Y, Kurosumi M, Natori T. Spontaneous long-term hyperglycemic rat with diabetic complications, OLETF strain. Diabetes. 1992. 42:1422–1428.
17. Richman PG, Meister A. Regulation of gamma-glutamyl-cysteine synthetase by nonallosteric feedback inhibition by glutathione. J Biol Chem. 1975. 250:1422–1426.
18. Sen CK, Packer L. Antioxidant and redox regulation of gene transcription. FASEB J. 1996. 10:709–720.
19. Ginn-Pease ME, Whisler RL. Optimal NF kappa B mediated transcriptional responses in Jurkat T cells exposed to oxidative stress are dependent on intracellular glutathione and costimulatory signals. Biochem Biophys Res Commun. 1996. 226:695–702.
20. Arrigo AP. Gene expression and the thiol redox state. Free Radic Biol Med. 1999. 27:936–944.
21. Tran PO, Parker SM, LeRoy E, Franklin CC, Kavanagh TJ, Zhang T, Zhou H, Vliet P, Oseid E, Harmon JS, Robertson RP. Adenoviral overexpression of the glutamylcysteine ligase catalytic subunit protects pancreatic islets against oxidative stress. J Biol Chem. 2004. 279:53988–53993.
22. Urata Y, Yamamoto H, Goto S, Tsushima H, Akazawa S, Yamashita S, Nagataki S, Kondo T. Long exposure to high glucose concentration impairs the responsive expression of gamma-glutamylcysteine synthetase by interleukin-1beta and tumor necrosis factor-alpha in mouse endothelial cells. J Biol Chem. 1996. 271:15146–15152.
23. Seltzer HS. Blood glutathione in mild diabetes mellitus before treatment and during sulfonylurea-induced hypoglycemia. Proc Soc Exp Biol Med. 1957. 95(1):74–76.
24. Chari SN, Nath N, Rathi AB. Glutathione and its redox system in diabetic polymorphonuclear leukocytes. Am J Med Sci. 1984. 287:14–15.
25. Thomas G, Skrinska V, Lucas FV, Schumacher OP. Platelet glutathione and thromboxane synthesis in diabetes. Diabetes. 1985. 34:951–954.
26. Raharjo S, Sofos JN, Schmitt GR. Solid phase acid extraction improves thiobarbituric acid methods to determine lipid oxidation. J Food Sci. 1993. 58:921–932.
27. Belch JJ, Mackay IR, Hill A, Jening SP. Oxidative stress is present in atherosclerotic peripheral arterial disease and further increased by diabetes mellitus. Int-Angiol. 1995. 14:385.
28. Wander RC, Du SH, Ketchum SO, Rowe KE. alpha-Tocopherol influences in vivo induce of lipid peroxidation in post menopausal woman given fish oil. J Nutr. 1996. 126:643–652.
29. Goldstein JL, Brown MS. Molecular medicine: the cholesterol quartet. Science. 2001. 292:1310–1312.
30. Mertens A, Holvoet P. Oxidized LDL and HDL: antagonists in atherothrombosis. FASEB J. 2001. 15:2073–2084.
31. Ross R. Cell biology of atherosclerosis. Annu Rev Physiol. 1995. 57:791–804.
32. Lu SC, Bao Y, Huang ZZ, Sarthy VP, Kannan R. Regulation of gamma-glutamylcysteine synthetase subunit gene expression in retinal Muller cells by oxidative stress. Invest Ophthalmol Vis Sci. 1999. 40:1776–1782.
33. Catherwood MA, Powell LA, Anderson P, McMaster D, Sharpe PC, Trimble ER. Glucose-induced oxidative stress in mesangial cells. Kidney Int. 2002. 61:599–608.
34. Tachi Y, Okuda Y, Bannai C, Okamura N, Bannai S, Yamashita K. High concentration of glucose causes impairment of the function of the glutathione redox cycle in human vascular smooth muscle cells. FEBS Lett. 1998. 421:19–22.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download