Abstract
Background
Adipose tissue produces and releases a variety of proinflammatory cytokines. The aim of this study was to investigate whether proinflammatory cytokines are increased and insulin resistance is presented in nonobese women with high body fat and low fat free mass.
Methods
Sixty nonobese adult premenopausal women (body mass index, BMI < 25 kg/m2) were included in this study. Body composition was determined by dual energy absoprtiometry (DXA). Fasting glucose, lipid profiles, tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), C reactive protein (CRP) and basal insulin were measured.
Results
The subjects with high body fat (≥ 30%) had higher CRP levels (P = 0.024), IL-6 levels (P = 0.008), insulin levels (P = 0.003), and homeostasis model assessment-IR (HOMA-IR) (P = 0.020) than those of the subjects with low body fat. In a subset of 32 subjects with high body fat (≥ 30%), the number of subjects with high fat free mass index (FFMI) (≥ 13.5 kg/m2) had higher atherogenic index than that of subjects with low FFMI (FFMI < 13.5 kg/m2) (P < 0.05). IL-6 was correlated with % body fat, fat mass index (FMI), and fat mass (P < 0.05). HOMA-IR was correlated with % body fat and FMI (P < 0.05). To investigate predictors of cytokines and HOMA-IR, multiple regression analysis was used. % body fat was a predictor for IL-6 and, while age and % body fat were predictors of HOMA-IR in study subjects.
Figures and Tables
Fig. 2
Correlations between homeostasis model assessment IR. (HOMA-IR) and anthropometric data in study subjects.

Table 1
Comparisons of serum lipid profiles, fasting glucose and insulin, HOMA-IR, CRP, TNF-α, and IL-6 according to % body fat
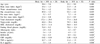
Table 2
Comparisons of serum lipid profiles, fasting blood glucose, CRP, TNF-a, and IL-6 according to body fat % and fat free mass index (FFMI)

※P < 0.05 and ※※P < 0.01; group 1 vs group 2, #P < 0.05 and ##P < 0.01; group 2 vs group 3, ∮P < 0.05 and ∮∮P < 0.01 group 1 vs group 3, †P < 0.05 and ‡P < 0.01; group 3 vs group 4, §P < 0.05 and §§P < 0.01 group 2 vs group 4 by Mann Whitney U-test, ∫P < 0.05 and ∫∫P < 0.01; group 1 vs group 4, and by General linear model after adjusted for age.
CRP, c-reactive protein; HDL, high density lipoprotein; HOMA-IR, homeostasis model assessment IR; IL-6, interleukin-6; TNF-α, tumor necrosis factor-α.
References
1. Castaneda C, Janssen I. Ethnic comparisons of sarcopenia and obesity in diabetes. Ethn Dis. 2005. 15:664–670.
2. Pedersen M, Bruunsgaard H, Weis N, Hendel HW, Andreassen BU, Eldrup E, Dela F, Pedersen BK. Circulating levels of TNF-alpha and IL-6-relation to truncal fat mass and muscle mass in healthy elderly individuals and in patients with type-2 diabetes. Mech Ageing Dev. 2003. 124:495–502.
3. Festa A, Ralph DA, Howard G, Mykkanen L, Tracy RP, Haffner SM. Chronic subclinical inflammation as part of the insulin resistance syndrome-The insulin resistance atherosclerosis study(IRAS). Circulation. 2000. 102:42–47.
4. Chambers JC, Cda S, Bassett P, Karim Y, Thompson SG, Gallimore JR, Pepys MB, Kooner JS. C-reactive protein, insulin resistance, central obesity, and coronary heart disease risk in indians from the United Kingdom compared with European whites. Circulation. 2001. 104:145–150.
5. Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-a: direct role in obesity-linked insulin resistance. Science. 1993. 259:87–89.
6. Reid MB, Li YP. Tumor necrosis factor-alpha and muscle wasting: a cellular perspective. Respir Res. 2001. 2:269–272.
7. Villareal DT, Banks M, Siener C, Sinacore DR, Klein S. Physical frailty and body composition in obese elderly men and women. Obes Res. 2004. 12:913–920.
8. Smalley KJ, Knerr AN, Kendrick ZV, Colliver JA, Owen OE. Reassessment of body mass indices. Am J Clin Nutr. 1990. 52:405–408.
9. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985. 28:412–419.
11. Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005. 96:939–949.
12. Wilson AM, Ryan MC, Boyle AJ. The novel role of C-reactive protein in cardiovascular disease: Risk marker or pathogen. Int J Cardiol. 2006. 106:291–297.
13. Bhagat K, Vallance P. Inflammatory cytokines impair endothelium-dependent dilatation in human veins in vivo. Circulation. 1997. 96:3042–3047.
14. Heinrich PC, Castell JV, Andus T. Interleukin-6 and the acute phase response. Biochem J. 1990. 265:621–636.
15. Vgontzas AN, Papanicolaou DA, Bixler EO, Kales A, Tyson K, Chrousos GP. Elevation of plasma cytokines in disorders of excessive daytime sleepiness: role of sleep disturbance and obesity. J Clin Endocrinol Metab. 1997. 82:1313–1316.
16. Panagiotakos DB, Pitsavos C, Yannakoulia M, Chrysohoou C, Stefanadis C. The implication of obesity and central fat on markers of chronic inflammation: The ATTICA study. Atherosclerosis. 2005. 183:308–315.
17. Fried SK, Bunkin DA, Greenberg AS. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab. 1998. 83:847–850.
18. Sewter CP, Digby JE, Blows F, Prins J, O'Rahilly S. Regulation of tumour necrosis factor-alpha release from human adipose tissue in vitro. J Endocrinol. 1999. 163:33–38.
19. Prins JB, Niesler CU, Winterford CM, Bright NA, Siddle K, O'Rahilly S, Walker NI, Cameron DP. Tumor necrosis factor-alpha induces apoptosis of human adipose cells. Diabetes. 1997. 46:1939–1944.
20. Dandona P, Aljada A, Chaudhuri A, Mohanty P, Garg R. Metabolic syndrome: a comprehensive perspective based on interactions between obesity, diabetes, and inflammation. Circulation. 2005. 111:1448–1454.
21. Karakelides H, Sreekumaran Nair K. Sarcopenia of aging and its metabolic impact. Curr Top Dev Biol. 2005. 68:123–148.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download