Abstract
Background
Islet transplantation is one of regimens supplying the deficient insulin in diabetes patients, but the effects of islet grafts on the changes of endogenous β-cells are not clear. In the present study, we examined the changes of endogenous β-cell mass after islet transplantation in partially pancreatectomized mice.
Methods
Balb/c mice were 70% pancreatectomized, transplanted with syngeneic islets (group IV), and were compared with pancreatectomized mice treated with insulin (group III) or no insulin (group II). Blood glucose levels and body weight were monitored. Remnant pancreas was obtained at 6 or 10 days after pancreatectomy, and immunohistochemical staining was done for the evaluation of β-cell mass changes.
Results
Hyperglycemia and weight loss were induced after pancreatectomy. After islet transplantation or insulin treatment, blood glucose levels recovered to normal, and body weight started to increase. Plasma insulin levels were higher and β-cell mass was larger in group IV than in group II (P < 0.05). Especially, the difference of β-cell mass between them was more evident at 7 days as compared to at 3 day after transplantation. When compared to group III, group IV showed larger individual β-cell area after 7 days and larger β-cell mass after 3 days of islet transplantation (P < 0.05).
Figures and Tables
Fig. 1
Changes in blood glucose levels and body weight.
Balb/c mice aged 10~12 weeks were allocated to 4 groups: sham operation (sham OP, I) 70% pancreatectomy (Px, II) 70% pancreatectomy with subsequent insulin treatment (Px + insulin, III) and 70% pancreatectomy with islet transplantation (Px + ITx, IV). Group II showed abrupt hyperglycemia and weight loss after Px, but the weight was stationary since 3 days after operation (open square). In group III (triangle) and IV (solid square), hyperglycemia developed after Px, but hyperglycemia became euglycemic with treatment (Tx: insulin administration or islet transplantation), and body weight started to recover after then.

Fig. 2
Intraperitoneal glucose tolerance test (IPGTT) after Px.
(A) At 6 days after Px (3 days after Tx), IPGTT was performed in each group. The pattern of glucose levels was similar in group III (triangle) and IV (solid square), however, the patterns of group III and IV were intolerant compared to group I (open circle).
(B) At 10 days after Px (7 days after Tx), group IV showed comparable results with group I. However, group III showed less glucose tolerance, and the difference with group IV was definite at that time.

Fig. 3
Fasting plasma insulin concentrations after Px.
Fasting plasma insulin was measured before and after operation with/without treatment. Subject number of each group (n) is shown on the graph. Insulin concentrations decreased to 10% at 3 days after Px. They increased at 6 days after Px (at 3 days after Tx). Although those of group IV tended to be higher than other groups, no statistical difference was observed. At 10 days after Px (at 7 days after Tx) insulin levels in group IV were higher than in group II and III, by more than two times (P < 0.005).
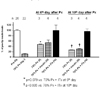
Fig. 4
Cell proliferation in remnant pancreas after Px.
(A) BrdU was injected before the mice were sacrificed, and then anti-BrdU staining was done with the remnant pancreas tissue. Subject number of each group (n) is shown on the graph. BrdU-positive cells were more prevalent in all the Px groups than in group I. At 6 days after Px (at 3 days after Tx), cell proliferation index was higher in group II and IV than in group III. At 10 days after Px (at 7 days after Tx), BrdU-positive cells were significantly higher in group III and IV than in group II. However, they were relatively decreased compared to each counterpart at 6 days after Px. Therefore, it suggests that cell proliferation is more prominent within 10 days after Px.
(B) Remnant pancreas tissues sections from group II ~ IV showing BrdU-positive cells indicate that cell proliferation occurred in all part of the pancreas, namely, islet, duct and exocrine tissue. However, it was not prominent in islets.

Fig. 5
Individual β-cell area in remnant pancreas.
Remnant pancreas tissue from each group was stained with anti-insulin antibody and individual β-cell area was measured as described in method section. It was increased after Px. At 6 days after Px (at 3 days after Tx), it was similar among group II, III and IV. At 10 days after Px (at 7 days after Tx), the increase progressed. Especially, the β-cell area was largest in group IV (P < 0.05).

Fig. 6
β-cell amount in remnant pancreas.
(A) Relative β-cell volume was calculated by point counting as described in method section. It increased after Px compared to group I. At 6 days after Px (at 3 days after Tx), the volume was larger in group III than in II, and larger in group IV than in III. Although these tendency was maintained At 10 days after Px (at 7 days after Tx), the statistical
significance between group III and IV was disappeared.
(B) Absolute β-cell mass was calculated with relative β-cell volume and weight of remnant pancreas. The pattern among group II~IV was similar with relative β-cell volume as in figure 6(A). β-cell mass in group IV at 10 days after Px (at 7 days after Tx) was increased upto 80% of group I.
(C) Insulin-positive cells in group IV (70% Px + ITx) were located not only in the islets but also in the exocrine and pancreatic duct (arrow). The upper panel was obtained from remnant pancreatic exocrine tissue, and the lowers were from the resection margin where active regeneration occurred.
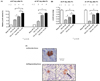
Fig. 7
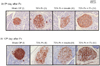
Intensity of insulin staining within islets.
Remnant pancreas tissue from each group was stained with anti-insulin antibody and the islets are presented. After Px, staining intensity became faint and uneven, which indicates insulin content in the islets were decreased. The degree of staining intensity was Sham OP (I), 70% Px + ITx (IV), 70% Px (II), and 70% Px + insulin (III) in the order named.
References
1. Bouwens L, Rooman I. Regulation of pancreatic beta-cell mass. Physiol Rev. 2005. 85:1255–1270.
2. Navarro X, Sutherland DE, Kennedy WR. Long-term effects of pancreatic transplantation on diabetic neuropathy. Ann Neurol. 1997. 42:727–736.
3. Fioretto P, Steffes MW, Sutherland DE, Goetz FC, Mauer M. Reversal of lesions of diabetic nephropathy after pancreas transplantation. N Engl J Med. 1998. 339:69–75.
4. Fiorina P, Folli F, Zerbini G, Maffi P, Gremizzi C, Di Carlo V, Socci C, Bertuzzi F, Kashgarian M, Secchi A. Islet transplantation is associated with improvement of renal function among uremic patients with type I diabetes mellitus and kidney transplants. J Am Soc Nephrol. 2003. 14:2150–2158.
5. Korsgren O, Jansson L, Andersson A. Effects of hyperglycemia on function of isolated mouse pancreatic islets transplanted under kidney capsule. Diabetes. 1989. 38:510–515.
6. Kilpatrick ED, Robertson RP. Differentiation between glucose-induced desensitization of insulin secretion and beta-cell exhaustion in the HIT-T15 cell line. Diabetes. 1998. 47:606–611.
7. Leahy JL, Bonner-Weir S, Weir GC. Beta-cell dysfunction induced by chronic hyperglycemia. Current ideas on mechanism of impaired glucose-induced insulin secretion. Diabetes Care. 1992. 15:442–455.
8. Montana E, Bonner-Weir S, Weir GC. Beta cell mass and growth after syngeneic islet cell transplantation in normal and streptozocin diabetic C57BL/6 mice. J Clin Invest. 1993. 91:780–787.
9. Juang JH, Bonner-Weir S, Wu YJ, Weir GC. Beneficial influence of glycemic control upon the growth and function of transplanted islets. Diabetes. 1994. 43:1334–1339.
10. Hamamoto Y, Tsuura Y, Fujimoto S, Nagata M, Takeda T, Mukai E, Fujita J, Yamada Y, Seino Y. Recovery of function and mass of endogenous beta-cells in streptozotocin-induced diabetic rats treated with islet transplantation. Biochem Biophys Res Commun. 2001. 287:104–109.
11. Plachot C, Movassat J, Portha B. Impaired beta-cell regeneration after partial pancreatectomy in the adult Goto-Kakizaki rat, a spontaneous model of type II diabetes. Histochem Cell Biol. 2001. 116:131–139.
12. Miao G, Ito T, Uchikoshi F, Tanemura M, Kawamoto K, Shimada K, Nozawa M, Matsuda H. Beneficial effects of pancreas transplantation: regeneration of pancreatic islets in the spontaneously diabetic Torii rat. Transplant Proc. 2005. 37:226–228.
13. Movassat J, Portha B. Beta-cell growth in the neonatal Goto-Kakisaki rat and regeneration after treatment with streptozotocin at birth. Diabetologia. 1999. 42:1098–1106.
14. Yamamoto M, Jia DM, Fukumitsu K, Otsuki M. Treatment for hyperglycemia promotes pancreatic regeneration in rats without CCK-1 receptor gene expression. Pancreas. 2003. 26:368–374.
15. Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004. 429:41–46.
16. Seaberg RM, Smukler SR, Kieffer TJ, Enikolopov G, Asghar Z, Wheeler MB, Korbutt G, van der Kooy D. Clonal identification of multipotent precursors from adult mouse pancreas that generate neural and pancreatic lineages. Nat Biotechnol. 2004. 22:1115–1124.
17. Suzuki A, Nakauchi H, Taniguchi H. Prospective isolation of multipotent pancreatic progenitors using flow-cytometric cell sorting. Diabetes. 2004. 53:2143–2152.
18. Gershengorn MC, Hardikar AA, Wei C, Geras-Raaka E, Marcus-Samuels B, Raaka BM. Epithelial-to-mesenchymal transition generates proliferative human islet precursor cells. Science. 2004. 306:2261–2264.
19. Kulkarni RN, Bruning JC, Winnay JN, Postic C, Magnuson MA, Kahn CR. Tissue-specific knockout of the insulin receptor in pancreatic beta cells creates an insulin secretory defect similar to that in type 2 diabetes. Cell. 1999. 96:329–339.
20. Serradas P, Bailbe D, Blondel O, Portha B. Abnormal B-cell function in rats with non-insulin-dependent diabetes induced by neonatal streptozotocin: effect of in vivo insulin, phlorizin, or vanadate treatments. Pancreas. 1991. 6:54–62.
21. Zhu M, Noma Y, Mizuno A, Sano T, Shima K. Poor capacity for proliferation of pancreatic beta-cells in Otsuka-Long-Evans-Tokushima Fatty rat: a model of spontaneous NIDDM. Diabetes. 1996. 45:941–946.
22. Ogino T, Zhu M, Murakami T, Kuwajima M, Shima K. Effect of partial pancreatectomy on beta-cell mass in the remnant pancreas of Wistar fatty rats. J Med Invest. 1998. 45:103–110.
23. Jonas JC, Sharma A, Hasenkamp W, Ilkova H, Patane G, Laybutt R, Bonner-Weir S, Weir GC. Chronic hyperglycemia triggers loss of pancreatic beta cell differentiation in an animal model of diabetes. J Biol Chem. 1999. 274:14112–14121.
24. Bonner-Weir S, Weir GC. New sources of pancreatic beta-cells. Nat Biotechnol. 2005. 23:857–861.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download