Abstract
Background
Because of the shortage of human pancreas and immunorejection, very small fraction of patients with type 1 diabetes can be treated with islet transplantation. The immune tolerance induction for overcoming the immume rejectin of trausplamted islets could be conducted by hematopoietic mixed chimerims with various invasive methods. The purpose of this study is to investigate the effect of mixed chimerims conducted by newly developed minimally invasive methods on islet allografts rejection in streptozotocin induced diabetic mice.
Methods
Recipient, Balb/c(H-2Kd) mice were injected intraperitoneally with anti-asialoGM1 antibody at one day before bone marrow transplantation. There were received total body irradiation at a dose of 500 cGy and followed by tail vein injection of the 2×107 T-cell depleted bone marrow cells from C57BL/6(H-2Kb). Mixed chimerism mice were determined by gDNA PCR of lymphocyte MHC class I gene (H-2K) on 21st day. Streptozotocin induced diabetic mixed chimera mice were received islet transplantation from bone marrow donors. Grafts, spleen and peripheral blood were obtained from the mixed chimera mice, and there were used by Immunohistochimeical staining, flow cytometric analysis and gDNA PCR on 21st day.
Results
The blood glucose levels of streptozotocin induced diabetic mice were normalized by transplantation of bone marrow donor islets and maintained during 30 days. After removal of first islet allografts, hyperglycemia was re-established. We could re-confirmed donor specific tolerance of transplanted islets by second transplantation of bone marrow donor islets. Normoglycemia was maintained during 21 days after second islet transplantation. Furthermore islet grafts from MHC-mismatched third party mice were immediately rejected. Flow cytometric analysis results suggest that the mixed chimerism mice were maintain during the whole study period.
Figures and Tables
Fig. 1
Schematic of experimental design. (A) Method of mixed chimerism mice induction. -1 day. Anti-asialoGM1 was injected to Balb/c. Total body irradiated and 2×107 Bone marrow cells were transplanted to the Balb/c on 0 day. (B) Islet transplantation schedule in STZ induced type 1 DM mixed chimerism mice. On 21 days before islets transplantation to induced mixed chimerism in Balb/c. On 6day before transplantation, type 1 diabetes was induced by STZ injection. On day 0. 1st B6 islets transplantation to the mixed chimerism mice. Graft removal or sham op was done on 30th day. On the 35th day, the second islet transplantation was performed, at the same time third party islets were co-transplanted on the other side of the same kidney. Finally, on the 56th day and the 65th day, all grafts, spleen, peripheral blood and pancreas were harvested.
TBI: Total body irradiation
BMCs: Bone marrow cells
BMT: Bone marrow transplantation
STZ: Streptozotocin
DM: Diabetes mellitus

Fig. 2
Pancreatic damage after streptozotocin treatment. Insulin immunohistochemical staining(Arrow) in wild Balb/c(B) and mixed chimerism Balb/c(A) pancreatic islet on 1 day before islet transplantation. BrdU immunohistochemical staining in wild Balb/c(D) and mixed chimerism Balb/c(C). pancreatic islet on 6th day after STZ treatment. Insulin immunohistochemical staining in Mixed chimerism Balb/c(E). on 65th day after islet transplanted of bone marrow cell donor. Beta cell percentage in islets of wild Balb/c and mixed chimerism Balb/c on -1 day(F). (Original magnification ×400, * P≤0.001)
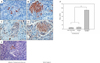
Fig. 4
Blood glucose levels were measured after islet transplantation. Islets of bone marrow cell donor were transplanted to STZ-induced diabetic mixed chimerims Balb/c. Islet of bone marrow cell donor and third party were re-transplanted after depleted(30th day) of the first graft(-●- 1st graft depleted group n=4, -○- 1st graft did not deplete group n=2). Islets of bone marrow cell donor were transplanted to wild Balb/c mice(-▼- n=14)
*P≤0.001 (-●- vs -▼- & -○- vs -▼-)
†Streptozotocin injection (300mg/Kg)
‡Islet transplantation
§IP-GTT(-●- & -○-)
∥End point of death(-▼-)

Fig. 5
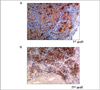
Insulin immunohistochemical staining in islet graft. (A) Islet of bone marrow cell donor in the graft was stained with insulin immunohistochemical staining at 56th day. (B) Second islet 1st graft depleted group were stained with insulin immunohistochemical staining at 26 days after 2nd islet transplantation. (Original magnification ×400)
References
1. Atkinson MA, Maclaren NK. The pathogenesis of insulin-dependent diabetes mellitus. N Engl J Med. 1994. 331(21):1428–1436.
2. Patricia M Graves, George S Eisenbarth. Pathogenesis, prediction and trials for the prevention of insulin-dependent (type 1) diabetes mellitus. Advanced Drug Delivery Reviews. 1999. 35:143–156.
3. Stratta R. Late acute rejection after pancreas transplantation. Transplantation Proceedings. 2000. 30:646.
4. Nakhleh RE, Gruessner RWG. Ischemia due to vascular rejection causes islet loss after pancreas transplantation. Transplantation Proceedings. 1998. 30:539–540.
5. Spiros Delis, Gaetano Ciancioa C, George W Burke III, Rolando Garcia-Morales, Joshua Miller. Donor bone marrow transplantation chimerism and tolerance. Transplant Immunology. 2004. 13:105–115.
6. kolb Hans-jochem, ledderose georg. Tolerance and chimerism. Transplantati. 2003. 75:26s–31s.
7. Li Hua, Inverardi Luca, Damaris R Molano, Antonello Pileggi, Camillo Ricordi. Nonlethal condiioning for the induction of allogeneic chimerism and tolerance to islet allografts. Transplantation. 2003. 75:966–970.
8. Zhiguang Guo, Tao Wu, Hakan Sozen, Yisheng Pan, Neal Heuss, Hannes Kalscheuer, Sutherland David ER, Blazar Bruce R, Hering Bernhard J. A substantial level of donor hematopoietic chimerism is required to protect donor=specific islet grafts in diabetic nod mice. Transplantation. 2003. 75:909–915.
9. Murphy WilliamJ, Kumar Vinay, Bennett Michael. Acute rejection of murine vone marrow allografts by natural killer cells and T cells-Differences in kinetic and Target antigen recognized. J Exp Med. 1987. 166:1499–1509.
10. Tiberghien Pierre, Longo Dan L, Wine John W, Alvord W Gregory, Reynolds Craig W. Anti-asialo GM1 antiserum treatment of lethally irradiated recipients before bone marrow transplantation: Evidence that recipient natural killer depletion enhances survival, engraftment, and hematopoietic recovery. Blood. 1990. 76:1419–1430.
11. Murphy William J, Koh Crystal Y, Raziuddin Arati, Bennett Michael, Longo Dan L. Immunobiology of natural killer cells and bone marrow transplantation: Merging of basic and preclinical studies. Immunological Reviews. 2001. 181:279–289.
12. Cho Seok Goo, Shuto Yukinobu, Soda Yasushi, Nakazaki Yukoh, Izawa Kiyoko, Uchimaru Kaoru, Takahashi Satoshi, Tani Kenzaburo, Tojo Arinobu, Asano Shigetaka. Anti-NK cell treatment induces stable mixed chimerism in MHC-mismatched, T cell-depleted, nonmyeloablative bone marrow transplantation. Exp Hematol. 2004. 32:1246–1254.
13. Weibel ER. Stereplogic methods. practical methods for biologic morphometry. 1978. 1. London: Academic Press;101–161.
14. Drachenberg Cinthia B, Klassen David K, Weir Matthew R, Wiland Ann, Fink Jeffrey C, Bartlett Stephen T, Cangro Charles B, Blahut Steven, Papadimitriou John C. Islet cell damage associated with tacrolimus and cyclosporine: Morphological features in pancreas allograft biopsieds and clinical correlation. Transplantation. 1999. 68:396–402.
15. Guo Zhiguang, Chong Anita SF, Shen Jikun, Foster Preston, Sankary Howard N, McChesney Lawrence, Mital Deepak, Jensik Stephen C, Gebel Howard, Williams James W. In vivo effects of leflunomide on normal pancreatic islet and syngeneic islet graft function. Transplantation. 1997. 63:716–721.
16. Russell Paul S, Chase Catharine M, Sykes Megan, Ito Hiroshi, Shaffer Juanita, Colvin Robert B. Tolerance, mixed chimerism, and chronic transplant arteriopathy. J Immunol. 2001. 167:5731–5740.
17. Sykes M. Mixed chimerism and transplant tolerance. Immunity. 2001. 14:417–424.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download