Abstract
Background
Islet transplantation is an alternative potential strategy to cure type 1 diabetes mellitus. However, two or more donors are usually needed for one recipient because a substantial part of the graft becomes nonfunctional due to several factors including hypoxia. Though hypoxic exposure of pancreatic beta cells has been reported to induce apoptotic cell death, the molecular processes involved in hypoxia-induced cell death are poorly understood. In type I diabetes, Nitric Oxide (NO) is known as an important cytokine, involved in the pathogenesis of beta cell dysfunction. Pancreatic beta cells are sensitive to the induction of inducible nitric oxide synthase (iNOS) when stimulated by TNF-α or IL-1β. But contribution of iNOS in response to hypoxia is not yet fully understood.
Methods
Mouse insulinoma cells (MIN6) were incubated in an anaerobic chamber (75% N2/15% CO2/5% H2) for up to 12 hours. Cell viability was measured after AO/PI staining. Caspase-3 activation was also determined using Western blot analysis. Nitric Oxide (NO) release into culture medium was measured using a Griess reagent. The expression of iNOS and PDX-1 mRNA and iNOS protein was examined using real time PCR and Western blot analysis.
Results
Marked cell death was observed within 6 hours after hypoxic exposure of MIN6 cells (control, < 5%; 2 hr, 11.0 ± 7.6%; 6 hr, 46.2 ± 12.8%, P < 0.05). Immunoreactivity to activated caspase-3 was observed at 2, 4 and 6 hrs. NO production was increased in a time dependent manner. Expression of iNOS mRNA and protein was significantly increased at 4 and 6 hour after hypoxia. iNOS expression was confirmed by immunostaining. Of note, Pdx-1 mRNA expression was markedly attenuated by hypoxic treatment. Pretreatment with a selective iNOS inhibitor, 1400 W, significantly prevented beta cell death induced by hypoxic injury.
Conclusion
Our data suggest that iNOS-NO play an important role in hypoxic injury to MIN6 cells. Therefore, iNOS-NO might be a potential therapeutic target for improving engraftment of the transplanted islets and suppression of iNOS would be helpful for prevention of beta cells damage to hypoxic injury.
Figures and Tables
Fig. 1
(A) Under hypoxic condition, oxygen concentration in culture media dropped to 20 mmHg within 2 hr. (B) AO/PI staining of the MIN6 cells showed remarkable cell death in time dependent manner. Six hours after anaerobic culture, cell death rate of MIN6 cells was more than 50%. (C) Effect of hypoxia on cell viability in cultured MIN6 cells using AO (Acridine orange, green) and PI (Propidium iodide, red) stain. Compared to normoxic condition, prominent cell death was demonstrated under anoxic culture for 2 and 6 h (a, normoxic condition; b, 2 hrs after anaerobic culture; c, 6 hrs after anaerobic culture. × 200).
*P < 0.05 vs. normoxic culture condition.
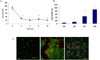
Fig. 2
Hypoxia-induced nitric oxide (NO) production, measured by accumulated nitrite concentration in culture medium. Hypoxia increased nitrite concentration in culture media in time-dependent manner in MIN6 cells. Nitrite production was measured using the Griess reagent method in the culture medium.
*P < 0.05 vs. in normoxic culture.

Fig. 3
Changes of the iNOS and PDX-1 mRNA expressions in response to hypoxic injury in MIN6 cells (A). Hypoxia increased iNOS mRNA expression (B) in the cultured MIN6 cells after 2 hrs. However, PDX-1 mRNA expression (C) was significantly decreased when they had been exposed to prolonged hypoxia.
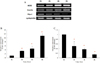
Fig. 4
Hypoxia induced the iNOS protein expression (130 kd) in MIN6 cells. The iNOS protein expression was detected at 2 h of hypoxic treatment and peaked at 4 h (A). But specific iNOS inhibitor (1400 W) pretreatment effectively attenuated the iNOS expression after hypoxic injury (B).
*P < 0.05 vs. normoxic culture.
**P < 0.05 vs. hypoxic treatment for 4 hrs without 1400 W.
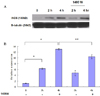
Fig. 5
Caspase-3 activity was significantly increased in response to hypoxic injury in MIN6 cells.
*P < 0.05 vs. normoxic culture.
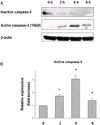
Fig. 6
DETA-NO (NO donor) treatment in MIN6 cells. 12 hrs After DETA-NO treatment in normoxic condition, PDX-1 mRNA expression was significantly decreased in dose-dependent manner without hypoxic injury.
*P < 0.05 vs. normoxic culture.
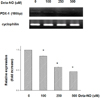
Fig. 7
iNOS and PDX-1 immunostaining after hypoxic culture in MIN6 cells. Compared to normixic culture condition (A), red-colored iNOS staining was remarkably increased after 4 hrs of hypoxic treatment (B). PDX-1 immunostaining (green) in the MIN6 cells showed that PDX-1 expression was significantly decreased after 4 hrs of hypoxic treatment (D), compared to normoxic cultured cells (C). Nuclear staining of PDX-1 was rarely seen at 4 hrs. Blue color; DAPI staining. A, B; × 400, C, D; × 1000.
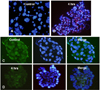
Fig. 8
This is cell viability using staining after 1400W treatment. Cell death rate at 2 and 6 hrs after hypoxic injury was significantly increased. But 1400W (specific iNOS inhibitor) pretreatment significantly decreased PI staining area at 2 and 6 hrs. 1400W pretreatment significantly attenuated the cell death rate.

References
1. Saaddine JB, Cadwell B, Gregg EW, Engelgau MM, Vinicor F, Imperatore G, Narayan KM. Improvements in diabetes processes of care and intermediate outcomes: United States, 1988-2002. Ann Intern Med. 2006. 144:525–527.
2. Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000. 343:230–238.
3. Weir GC. Can we make surrogate beta-cells better than the original? Semin Cell Dev Biol. 2004. 15:347–357.
4. Yoon KH, Ko SH, Cho JH, Lee JM, Ahn YB, Song KH, Yoo SJ, Kang MI, Cha BY, LeeKW , Son HY, Kang SK, Kim HS, Lee IK, Bonner-Weir S. Selective beta-cell loss and alpha-cell expansion in patients with type 2diabetes mellitus in Korea. J Clin Endocrinol Metab. 2003. 88:2300–2308.
5. Robertson RP. Islet transplantation as a treatment for diabetes - a work in progress. N Engl J Med. 2005. 350:694–705.
6. Barshes NR, Wyllie S, Goss JA. Inflammation-mediated dysfunction and apoptosis in pancreatic islet transplantation: implications for intrahepatic grafts. J Leukoc Biol. 2005. 77:587–597.
7. Linn T, Schmitz J, Hauck-Schmalenberger I, Lai Y, Bretzel RG, Brandhorst H, randhorst D. Ischaemia is linked to inflammation and induction of angiogenesis in pancreatic islets. Clin Exp Immunol. 2006. 144:179–187.
8. Kroncke KD, Fehsel K, Suschek C, Kolb-Bachofen V. Inducible nitric oxide synthase-derived nitric oxide in gene regulation, cell death and cell survival. Int Immunopharmacol. 2001. 1:1407–1420.
9. Beck KF, Eberhardt W, Frank S, Huwiler A, Messmer UK, Muhl H, Pfeilschifter J. Inducible NO synthase: role in cellular signalling. J Exp Biol. 1999. 202:645–653.
10. Corbett JA, Sweetland MA, Wang JL, Lancaster JR Jr, McDaniel ML. Nitric oxide mediates cytokine-induced inhibition of insulin secretion by human islets of Langerhans. Proc Natl Acad Sci U S A. 1993. 90:1731–1735.
11. Eizirik DL, Pavlovic D. Is there a role for nitric oxide in beta-cell dysfunction and damage in IDDM? Diabetes Metab Rev. 1997. 13:293–307.
12. Welsh N, Eizirik DL, Bendtzen K, Sandler S. Interleukin-1 beta-induced nitric oxide production in isolated rat pancreatic islets requires gene transcription and may lead to inhibition of the Krebs cycle enzyme aconitase. Endocrinology. 1991. 126:3167–3173.
13. Steller H. Mechanisms and genes of cellular suicide. Science. 1995. 267:1445–1449.
14. Henningsson R, Salehi A, Lundquist I. Role of nitric oxide synthase isoforms in glucose-stimulated insulin release. Am J Physiol Cell Physiol. 2002. 283:C296–C304.
15. Postovit LM, Sullivan R, Adams MA, Graham CH. Nitric oxide signalling and cellular adaptations to changes in oxygenation. Toxicology. 2005. 208:235–248.
16. Semenza GL. Hypoxia-inducible factor 1: oxygen homeostasis and disease pathophysiology. Trends Mol Med. 2001. 7:345–350.
17. Moritz W, Meier F, Stroka DM, Giuliani M, Kugelmeier P, Nett PC, Lehmann R, Candinas D, Gassmann M, Weber M. Apoptosis in hypoxic human pancreatic islets correlates with HIF-1alpha expression. FASEB J. 2002. 16:745–747.
18. Keinanen R, Vartiainen N, Koistinaho J. Molecular cloning and characterization of the rat inducible nitric oxide synthase (iNOS) gene. Gene. 1999. 234:297–305.
19. Dionne KE, Colton CK, Yarmush ML. Effect of hypoxia on insulin secretion by isolated rat and canine islets of Langerhans. Diabetes. 1993. 42:12–21.
20. Davalli AM, Scaglia L, Zangen DH, Hollister J, Bonner-Weir S, Weir GC. Vulnerability of islets in the immediate posttransplantation period. Dynamic changes in structure and function. Diabetes. 1996. 45:1161–1167.
21. Biarnes M, Montolio M, Nacher V, Raurell M, Soler J, Montanya E. Beta-cell death and mass in syngeneically transplanted islets exposed to short-and long-term hyperglycemia. Diabetes. 2002. 51:66–72.
22. Carlsson PO, Palm F, Andersson A, Liss P. Markedly decreased oxygen tension in transplanted rat pancreatic islets irrespective of the implantation site. Diabetes. 2001. 50:489–495.
23. Zagorska A, Jozef Dulak. HIF-1: the knowns and unknowns of hypoxia sensing. Acta Biochimica Polonica. 2004. 51:563–585.
24. McCabe C, Samali A, O'brien T. Beta cell cytoprotective strategies: establishing the relative roles for iNOS and ROS. Biochem Biophys Res Commun. 2006. 342:1240–1248.
25. Stoorling J, Binzer J, Andersson AK, Zullig RA, Tonnesen M, Lehmann R, Spinas GA, Sandler S, Billestrup N, Mandrup-Poulsen T. Nitric oxide contributes to cytokine-induced apoptosis in pancreatic beta cells via potentiation of JNK activity and inhibition of Akt. Diabetologia. 2005. 48:2039–2050.
26. Brune B, von Knethen A, Sandau KB. Nitric oxide and its role in apoptosis. Eur J Pharmacol. 1998. 351:261–272.
27. Beeharry N, Chambers JA, Faragher RG, Garnett KE, Green IC. Analysis of cytokine-induced NO-dependent apoptosis using RNA interference or inhibition by 1400W. Nitric Oxide. 2004. 10:112–118.
28. Rydgren T, Sandler S. Efficacy of 1400 W, a novel inhibitor of inducible nitric oxide synthase, inpreventing interleukin-1beta-induced suppression of pancreatic islet function invitro and multiple low-dose streptozotocin-induced diabetes in vivo. Eur J Endocrinol. 2002. 147:543–551.
29. Kato Y, Miura Y, Yamamoto N, Ozaki N, Oiso Y. Suppressive effects of a selective inducible nitric oxide synthase (iNOS) inhibitor on pancreatic beta-cell dysfunction. Diabetologia. 2003. 46:1228–1233.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download