Abstract
Background
The regulation of tyrosine phosphorylation/dephosphorylation is an important mechanism in various intracellular metabolism. Also impaired insulin signal transduction is important in pathogenesis of type 2 diabetes. It has been reported that PTP1B is a negative regulator of insulin action, and Giα2-protein is related to the regulation of PTP1B. Herein we investigated the long-term effects of ramipril on PTP1B/insulin signal protein interaction and the relation between Giα2 and PTP1B in animal model of type 2 diabetes (OLETF rat).
Methods
OLETF rats and age-matched LETO rats were divided into two groups. One group of rats received ramipril (10 mg/kg body weight) for 12 weeks, and another group did not. Finally, each group was divided into 2 subgroups, with or without insulin injection intravenously, before sacrifice. After sacrifice, tissues extracts of liver, hind limb muscle, and epididymal fat were obtained for quantification of PTP1B, Giα2, and several insulin signal proteins by western blotting.
Results
In liver and muscle, the levels of basal PTP1B and activated PTP1B of OLETF rats treated with ramipril and insulin were significantly decreased. The levels of Giα2, activated IRS-2, and activated p-85α were significantly increased in OLETF rats treated with ramipril and insulin. In adipose tissue, the levels of Giα2 and activated p-85α of OLETF rats treated with ramipril and insulin were slightly increased as in liver and muscle. But, the levels of basal PTP1B and activated PTP1B were significantly increased. And, the levels of activated IRS-1 and activated IRS-2 were decreased.
Conclusion
These results suggest that the improvement of insulin sensitivity by treatment with ramipril was related to the decreased level of activated PTP1B. Also, we could suggest that the changes of activated PTP1B level was related with the changes of Giα2-protein. However, the results of adipose tissue were different from those of liver and muscle. So it seemed likely that there would be various major modulators for regulation of insulin signal pathway according to tissue.
Figures and Tables
Fig. 1
The changes of body weight (A), and the differences of oral glucose tolerance test (B) of LETO and OLETF rats with or without ramipril treatment. Oral glucose tolerance tests were done at 12 weeks after ramipril treatment. Data were expressed as mean ± SE. *P < 0.05

Fig. 2
Effects of ramipril on tyrosine phosphorylation of PTP1B in liver (A), muscle (B), and fat tissue (C) of OLETF rats with or without ramipril treatment. The proteins were isolated with extraction buffer as described in methods and kept on ice. After centrifugation, aliquots of the supernatant were immunoprecipitated (IP) with anti-IR- and immunoblotted (IB) with anti-PTP1B antibodies (left panel of A, B, C), IP with anti-phosphotyrosine antibodies and IB with anti-PTP1B antibodies (right panel of A, B, C). Data were expressed as mean ± SE and represented by fold increase comparing with the control protein (#) that were normalized to 1. *P < 0.05
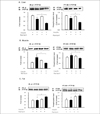
Fig. 3
Effects of ramipril on Giα2-protein in live r (A), muscle (B), and adipose tissue (C) of OLETF rats with or without ramipril treatment. The proteins were isolated with extraction buffer as described in methods and kept on ice. After centrifugation, aliquots of the supernatant were immunoblotted (IB) with anti-Giα2 antibodies (upper blot), and were immunoprecipitated (IP) with anti-phosphotyrosine antibodies and immunoblotted with anti-Giα2 antibodies (lower blot). Data were expressed as mean ± SE and represented by fold increase comparing with the control protein (#) that were normalized to 1. *P < 0.05
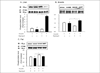
Fig. 4
Effects of ramipril on insulin-induced tyrosine phosphorylation of IR-, IRS-1, IRS-2, and p-85 in liver of OLETF rats with or without ramipril treatment. The proteins were isolated with extraction buffer as described in methods and kept on ice. After centrifugation, aliquots of the supernatant were immunoprecipitated (IP) with anti-IR- and immunoblotted (IB) with anti-IR-antibodies (A, upper blot), IP with anti-IR- and IB with anti-phosphotyrosine antibodies (A, lower blot), IP with anti-phosphotyrosine and IB with anti-IRS1 antibodies (B), IP with anti-phosphotyrosine and IB with IRS-2 antibodies (C), IP with anti-phosphotyrosine and IB with anti- p-85 antibodies (D). Data were expressed as mean ± SE and represented by fold increase comparing with the control protein (#) that were normalized to 1. *P < 0.05
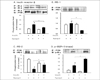
Fig. 5
Effects of ramipril on insulin-induced tyrosine phosphorylation of IR-, IRS-2, and p-85 in muscle of OLETF rats with or without ramipril treatment. Other procedures were similar in figure 4. Data were expressed as mean S.E., and represented by fold increase comparing with the control protein(#) that were normalized to 1. *P < 0.05
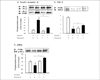
Fig. 6
Effects of ramipril on insulin-induced tyrosine phosphorylation of IR-, IRS-2, and p-85 in muscle of OLETF rats with or without ramipril treatment. Other procedures were similar in figure 4. Data were expressed as mean ± SE and represented by fold increase comparing with the control protein (#) that were normalized to 1. *P < 0.05
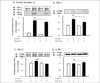
Fig. 7
Schematic representation of molecular mechanisms for insulin resistance (A) and improvement of insulin sensitivity by ramipril treatment (B). IR: insulin receptor, PI3-K: phosphatidylinositide-3 kinase, PTP1B: protein tyrosine phosphatase, IRS-1/-2: insulin receptor substrate-1/-2, PKA: protein kinase A.
References
1. Reaven GM. Role of insulin resistance in human disease. Diabetes. 1988. 37:1595–1607.
2. Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Inv. 2000. 106:473–481.
3. Saltiel AR. New perspectives into the molecular pathogenesis and treatment of type 2 diabetes. Cell. 2001. 104:517–529.
4. White MF, Kahn CR. The insulin signaling system. J Biol Chem. 1994. 269(1):1–4.
5. Goldstein BJ. Regulation of insulin receptor signaling by protein-tyrosine dephosphorylation. Receptor. 1993. 3:1–15.
6. Kenner KA, Anyanwu E, Olefsky JM, Kusari J. Protein-tyrosine phosphatase 1B is a negative regulator of insulin- and insulin-like growth factor-I-stimulated signaling. J Biol Chem. 1996. 271:19810–19816.
7. Egawa K, Maegawa H, Shimizu S, Morino K, Nishio Y, Bryer-Ash M, Cheung AT, Kolls JK, Kikkawa R, Kashiwagi A. Protein-tyrosine phosphatase 1B negatively regulates insulin signaling in L6 myocytes and Fao hepatoma cells. J Biol Chem. 2001. 276:10207–10211.
8. Gum RJ, Gaede LL, Koterski SL, Heindel M, Clampit JE, Zinker BA, Trevillyan JM, Ulrich RG, Jirousek MR, Rondinon CM. Reduction of protein tyrosine phosphatase 1B increase insulin-dependent signaling in ob/ob mice. Diabetes. 2003. 52:21–29.
9. Zabolotny JM, Haj FG, Kim HJ, Shulman GI, Kim JK, Neel BG, Kahn BB. Transgenic overexpression of PTP1B in muscle causes insulin resistance but overexpression with LAR does not additively impair insulin action. J Biol Chem. 2004. 279:24844–24851.
10. Ahmad F, Li PM, Meyerovitch J, Goldstein BJ. Osmotic loading of neutralizing antibodies demonstrates a role for protein-tyrosine phosphatase 1B In negative regulation of the Insulin action pathway. J Biol Chem. 1995. 270:20503–20508.
11. Kushner JA, Haj FG, Klaman LD, Dow MA, Kahn BB, Neel BG, White MF. Islet-sparing effects of protein tyrosine phosphatase-1b deficiency delays onset of diabetes in IRS2 Knockout mice. Diabetes. 2004. 53:61–66.
12. Tao J, Malbon CC, Wang HY. Giα2 enhances insulin signaling via suppression of protein-tyrosine phosphatase 1B. J Biol Chem. 2001. 276:39705–39712.
13. Hansson L, Lindholm LH, Niskanen L, Lanke J, Hedner T, Niklason A, Luomanmaki K, Dahlof B, de Faire U, Morlin C, Karlberg BE, Wester PO, Bjorck JE. Effects of angiotensin-converting-enzyme inhibition compare with conventional therapy on cardiovascular morbidity and mortality in hypertension: the Captopril Prevention Project(CAPP) randomized trial. Lancet. 1999. 353:611–616.
14. Yusuf S, Gerstein H, Hoogwerf B, Pogue J, Bosch J, Wolffenbuttel BH, Zinman B. Ramipril and the development of diabetes. JAMA. 2001. 286:1882–1885.
15. Dahlof B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, Faire U, Fyhrquist F, Ibsen H, Kristiansson K, Lederballe-Pedersen O, Lindholm LH, Nieminen MS, Omvik P, Oparil S, Wedel H. Cardiovascular morbidity and mortality in the losartan intervention for endpoint reduction in hypertensive study(LIFE): a randomized trial against atenolol. Lancet. 2002. 359:995–1003.
16. RAO RH. Pressor doses of angiotensin II increase hepatic glucose output and decrease insulin sensitivity in rats. J Endocrinol. 1996. 148(2):311–318.
17. Ogihara T, Asano T, Ando K, Chiba Y, Sakoda H, Anai M, Shojima N, Ono H, Onishi Y, Fujishiro M, Katagiri H, Fukushima Y, Kikuchi M, Noguchi N, Aburatani H, Komuro I, Fujita T. Angiotensin II-induced insulin resistance is associated with enhanced insulin signaling. Hypertension. 2002. 40:872–879.
18. Krutzfeldt J, Raasch W, Klein HH. Ramipril increases the protein level of skeletal muscle IRS-1 and alters protein tyrosine phosphatase activity in spontaneously hypertensive rats. Arch Pharmacol. 2000. 362:1–6.
19. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976. 72:248–256.
20. Moxham CM, Malbon CC. Insulin action impaired by deficiency of the G-protein subunit Giα2. Nature. 1996. 379:840–844.
21. Nawano M, Anai M, Funaki M, Kobayashi H, Kanda A, Fukushima Y, Inukai K, Ogihara T, Sakoda H, Onishi Y, Kikuchi M, Yazaki Y, Oka Y, Asano T. Imidapril, an angiotensin-converting enzyme inhibitor, improves insulin sensitivity by enhancing signal transduction via insulin receptor substrate proteins and improving vascular resistance in the Zuker Fatty rat. Metabolism. 1999. 48:1248–1255.
22. Sechi LA, Griffin CA, Zingaro L, Valentin JP, Bartoli E, Schambelan M. Effects of angiotensin II on insulin receptor binding and mRNA levels in normal and diabetic rats. Diabetologia. 1997. 40:770–777.
23. Ludvik B, Kueenburg E, Brunnbauer M, Schernthaner G, Prager R. The effects of ramipril on glucose tolerance, insulin secretion, and insulin sensitivity in patients with hypertension. J Cardiovasc Pharmacol. 1991. 18:Suppl 2. S157–S159.
24. Ukkola O, Santaniemi M. Protein tyrosine phosphatase 1B: a new target for the treatment of obesity and associated co-morbidities. Int Med. 2002. 251:467–475.
25. Tao J, Malbon CC, Wang HY. Insulin stimulates tyrosine phosphorylation and inactivation of protein-tyrosine phosphatase 1B in vivo. J Biol Chem. 2001. 276:29520–29525.
26. Elchebly M, Payette P, Michaliszyn E, Cromlish W, Collins S, Loy AL, Normandin D, Cheng A, Himms-Hagen J, Chan CC, Ramchandran C, Gresser MJ, Tremblay ML, Kennedy BP. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase 1B gene. Science. 1999. 283:1544–1548.
27. Heyworth CM, Houslay MD. Insulin exerts action through a distinct species of guanine nucleotide regulatory protein : inhibition of adenylate cyclase. Biochem J. 1983. 214:547–552.
28. Gawler D, Milligan G, Spiegel AM, Unson CG, Houslay MD. Abolition of the expression of inhibitory guanine nucleotide regulatory protein Gi activity in diabetes. Nature. 1987. 327:229–232.
29. Crespo P, de Mora JF, Aaronson DS, Santos E, Gutkind JS. Transforming G protein-coupled receptors block insulin and ras-induced adipocytic differentiation in 3T3-L1 cells: evidence for a PKC and MAP kinase independent pathway. Biochem Biophys Res Commun. 1998. 245:554–561.
30. Hart MJ, Jiang X, Kozasa T, Roscoe W, Singer WD, Gilman AG, Sternweis PC, Bollag G. Direct stimulation of the guanine nucleotide exchange activity of p115 RhoGEF by Galpha13. Science. 1998. 280:2112–2114.
31. Lefkowitz RJ. G protein-coupled receptors. III. New roles for receptor kinases and beta-arrestins in receptor signaling and desensitization. J Biol Chem. 1998. 273:18677–18680.
32. Wang L, Hayashi H, Kishi K, Huang L, Hagi A, Tamaoka K, Hawkins PT, Ebina Y. Gi-mediated translocation of GLUT4 is independent of p85/p110alpha and p110gamma phosphoinositide 3-kinases but might involve the activation of Akt kinase. Biochem J. 2000. 345:543–555.
33. Zheng XL, Guo J, Wang H, Malbon CC. Expression of constitutively activated Gialpha2 in vivo ameliorates streptozotocin-induced diabetes. J Biol Chem. 1998. 273:23649–23651.
34. Pandey SK, Anand-Srivastava MB. Modulation of G-protein expression by the angiotensin converting enzyme inhibitor captopril in heart from spontaneously hypertensive rats. AJH. 1996. 9:833–837.
35. Calera MR, Vallega G, Pilch PF. Dynamics of protein-tyrosine phosphatase In rat adipocytes. J Biol Chem. 2000. 275:6308–6312.
36. He W, Craparo A, Zhu Y, O'Neill TJ, Wang LM, Pierce JH, Gustafson TA. Interaction of insulin receptor substrate-2(IRS-2) with the insulin and insulin-like growth factor I receptor. Evidence for two distinct phosphotyrosine-dependent interaction domains within IRS-2. J Biol Chem. 1996. 271:11641–11648.
37. Sawka-Verhelle D, Tartare-Deckert S, White MF, Van Obberghen E. Insulin receptor substrate-2 binds to the insulin receptor through its phosphotyrosine-binding domain and through a newly identified domain comprising amino acids 591-786. J Biol Chem. 1996. 271:5983–5989.
38. Ogihara T, Shin BC, Anai M, Katagiri H, Inukai K, Funaki M, Fukushima Y, Ishihara H, Takata K, Kikuchi M, Yazak Y, Oka Y, Asano T. Insulin receptor substrate(IRS)-2 is dephosphorylated more rapidly than IRS-1 via its association with phosphatidylinositol 3-kinase in skeletal muscle cells. J Biol Chem. 1997. 272:12868–12873.
39. Rother KI, Imai Y, Caruso M, Beguinot F, Formisano P. Evidence that IRS-2 phosphorylation is required for insulin action in hepatocytes. J Biol Chem. 1988. 273:17491–17497.
40. Kubota N, Tobe K, Terauchi Y, Eto K, Yamauchi T, Suzuki R, Tsubamoto Y, Komeda K, Nakano R, Miki H, Satoh S, Sekihara H, Sciacchitano S, Lesniak M, Aizawa S, Nagai R, Kimura S, Akanuma Y, Taylor SI, Kadowaki T. Disruption of insulin receptor substrate 2 causes type 2 diabetes because of liver insulin resistance and lack of compensatory beta-cell hyperplasia. Diabetes. 2000. 49:1880–1889.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download