Abstract
Adverse reactions of subcutaneous low molecular weight heparin or unfractionated heparin could be complications by bleeding, heparin-induced thrombocytopenia, drug-induced liver injury, osteoporosis, and cutaneous reactions. Heparin-induced skin lesions vary from allergic reactions like erythema, urticaria, eczema to intradermal microvascular thrombosis associated with heparin-induced thrombocytopenia. There is a rare cutaneous complication, called bullous hemorrhagic dermatosis. We experienced this rare case of the cutaneous complication caused by enoxaparin. Several tense bullous hemorrhagic lesions occurred after 3 days of enoxaparin in a known bullous pemphigoid patient who had aortic valve replacement surgery with a mechanical prosthesis. The bullous hemorrhagic lesions were regressed after the discontinuation of enoxaparin but recurred after re-administration. The lesions were controlled by the administration of systemic corticosteroid and alternative anticoagulant. To date, less than 20 cases have been reported worldwide. This is the first case of bullous hemorrhagic dermatosis induced by enoxaparin, a low-molecular-weight heparin in Korea. This is also the first case of bullous hemorrhagic dermatosis in a known bullous pemphigoid patient.
Heparin is used for the prevention and treatment of venous thromboembolism. There are 2 types of heparins, unfractionated heparin and low-molecular-weight heparin. Both heparins act as the anticoagulant by activating antithrombin III [1]. However, low-molecular-weight heparin is more widely used than unfractionated heparin because its anticoagulant response is more predictable and stable so that it can be administered in fixed doses subcutaneously without laboratory monitoring [2].
There are some adverse effects of subcutaneous heparin such as bleeding complications, heparin-induced thrombocytopenia, drug-induced liver injury, osteoporosis, and cutaneous reactions [34]. Heparin-induced skin lesions vary from allergic reactions like erythema, urticaria, eczema to intradermal microvascular thrombosis associated with heparin-induced thrombocytopenia [3]. There is a rare cutaneous complication, called bullous hemorrhagic dermatosis [5]. To date, less than 20 cases have been reported worldwide. Here we report the first case of bullous hemorrhagic dermatosis induced by Enoxaparin, a low-molecular-weight heparin in Korea. This is also the first case of bullous hemorrhagic dermatosis in a known bullous pemphigoid patient.
A 75-year-old female patient visited the Emergency Department due to hypotension. She was on warfarin after aortic valve replacement surgery with a mechanical prosthesis for 26 years and in the state of right hemiplegia due to subacute subdural hematoma for 2 years. One year ago, multiple bullous lesions occurred on whole body, and the diagnosis of bullous pemphigoid was made after skin biopsy. The bullous lesions had never been hemorrhagic, and they had been well controlled with prednisolone 20 mg once a day and methotrexate 10 mg a week. Upon examination, she had a large hematoma on right buttock area and laboratory test revealed low hemoglobin, 7.1 g/dL and prolonged prothrombin time, >120 seconds, <10%, >17.8 international normalized ratio (INR). One week before visiting our hospital, generalized tonic-clonic seizures occurred and valproic acid was started by a neurologist. Because there was possibility of drug interaction between warfarin and valproic acid as a cause of increased prothrombin time, valproic acid discontinued. In addition, fresh-frozen plasma and vitamin K were administered and prothrombin level was normalized.
On the 10th day of hospitalization, enoxaparin sodium (40 mg every 12 hours by subcutaneous injection) was started for anticoagulation. Three days after starting enoxaparin therapy the patient developed several tense hemorrhagic bullae on distant sites: the right forearm, the dorsum of the right hand and the left foot. The most severe lesion was on the right forearm (Fig. 1A), 2 days later, bleeding occurred due to a rupture of hemorrhagic bulla (Fig. 1B). The patient was referred to an allergist. Bullous hemorrhagic dermatosis related to enoxaparin use was suspected. Enoxaparin was stopped from that day and readministrated with caution 4 days later after improvement of the hemorrhagic bullae because of the high risk of thromboembolic event due to the artificial valve and previously too prolonged prothrombin level. However, as hemorrhagic bulla worsened again, enoxaparin was discontinued. Methylprednisolone 40 mg (1 mg/kg) every 12 hours by intravenous injection was administered after skin biopsy.
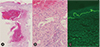
Laboratory test revealed a low level of hemoglobin (9.6 g/dL), normal platelet count (307,000/µL) and normal prothrombin time (61%, 16.8 seconds, 1.38 INR), and an elevated activated partial thromboplastin time (59.2 seconds). Results of serum biochemistry test were unremarkable. A punch biopsy was performed from the lesion over the right forearm and pathologic findings revealed increased vascular channels with hematoma formation and superficial perivascular lymphocytic and a few eosinophilic infiltration (Fig. 2A, B). There is no evidence of vasculitis or capillary thrombosis. Direct immunofluorescence showed that C3 was strongly positive at the dermoepidermal junction and this result may be due to the underlying bullous pemphigoid (Fig. 2C).
After the administration of high-dose steroid, the bullous hemorrhagic lesions were improved dramatically (Fig. 1C). Warfarin was administered for anticoagulation without any further problem. The hemorrhagic bulla regressed without sequelae. Methylprednisolone was administered 25 mg once a day because of the underlying bullous pemphigoid. The patient was discharged from the ninth week of hospitalization without any recurrence of hemorrhagic bullous lesions or bullous pemphigoid.
In 2006, Perrinaud et al. [6] reported three cases of bullous hemorrhagic dermatosis occurred after subcutaneous heparin injection. Since then, less than 20 cases have been reported worldwide. Bullous hemorrhagic dermatosis occurs at a distance from the heparin injection site and mainly occurs in limbs and abdomen [789]. Tense, hemorrhagic bullae occur without other accompanying symptoms such as itching sense, pain [10]. It is known that there is a temporal relationship between bullae development and subcutaneous heparin therapy, and bullae occur approximately 5 to 21 days after heparin administration [3]. In some cases, it was reported to occur 2 or 3 days later [7]. In our case, the patient developed multiple, tense, hemorrhagic bullae at the right forearm, the right hand and the left foot 3 days after starting enoxaparin. Although there are limited numbers of case reports, elderly could be one risk factor. The age of onset is reported from 51 to 91 years [10]. The patient of this case was also 75 years old.
Enoxaparin, a low-molecular-weight heparin, is the most common cause, and it is likely that enoxaparin is the most prescribed low-molecular-weight heparin [3]. Cases of hemorrhagic bullous dermatosis due to dalteparin, tinzaparin, and unfractionated heparin have been reported [711]. There could be cross-reactivity among low-molecular-weight heparin or unfractionated heparin but it is not clear. In most case reports, the authors reported that they used an alternative anticoagulant such as Warfarin. It has been reported that unfractionated heparin could be used in enoxaparin-induced bullous hemorrhagic dermatosis [12] and even that enoxaparin could be readministered without recurrence [11]. However, in this case, the bullous hemorrhagic lesions recurred after the readministration of enoxaparin. Severity could be one factor: our case was the most severe case with hemorrhagic rupture.
The pathogenesis of bullous hemorrhagic dermatosis is unknown while the most common cause of heparin-induced skin lesions is type 4 hypersensitivity or heparin-induced thrombocytopenia [13]. The bleeding tendency due to the anticoagulant effect is unlikely to be the cause of the skin lesion considering unremarkable platelet count and coagulation profiles.
Skin biopsy can distinguish bullous hemorrhagic dermatosis from other skin lesions [9]. Blood-filled intraepidermal or subcorneal vesicles and blisters with no signs of vasculitis or capillary thrombosis have been reported [11]. Infiltration of inflammatory cells such as eosinophil or neutrophil was not definite, and direct immunofluorescence test result is usually negative [8910]. Although we did not check antiplatelet factor 4/heparin antibodies in skin biopsy specimens, it has been reported that antiplatelet factor 4/heparin antibodies were usually negative [10]. In this case, pathologic findings revealed intradermal vesicle formation with filled red blood cells and superficial perivascular lymphocytic and a few eosinophilic infiltration without vasculitis or capillary thrombosis. Direct immunofluorescence showed that C3 was strongly positive at the dermoepidermal junction. The pathologic findings indicated the underlying bullous pemphigoid. Bullous pemphigoid could be hemorrhagic. However, in this patient, the bullous lesions of previously known bullous pemphigoid had never been hemorrhagic before. The small and large bullous hemorrhagic lesions induced by enoxaparin in this case were different from those of the previous bullous lesions of bullous pemphigoid starting from purpuric nodular bullae to tense hemorrhagic bullae. Biopsy was done at the largest lesion on right forearm. Sizes of reported bullous hemorrhagic dermatosis in the literature varies from small to large [71114] and the biopsy findings of large bullous hemorrhagic dermatosis have not been published [14]. As far as we know, this is the first case of bullous hemorrhagic dermatosis induced by enoxaparin with bullous pemphigoid.
There is no clear guideline for managing bullous hemorrhagic dermatosis [7] and heparin was discontinued in most cases. But in some cases, heparin was maintained for some reason and nonetheless, the lesions improved [910]. However, in our case, hemorrhagic bullous lesions are more severe than previously reported cases and bleeding due to rupture of bullae was clinically significant. Because hemorrhagic bullae were aggravated again after readministration of enoxaparin, we concluded that discontinuation of enoxaparin was essential for recovery in this patient. We also administered high-dose corticosteroid because hemorrhagic bullae were very severe, the patient had underlying bullous pemphigoid, and clinically type IV hypersensitivity reaction was suspected. After administration of high-dose steroid, the hemorrhagic bullous lesions improved dramatically. Although in most cases, discontinuation of heparin and supportive care alone improved hemorrhagic bulla, high-dose steroid should be considered in severe cases.
Early suspicion, discontinuation of enoxaparin, and proper management are most important steps in the treatment of this rare disease. This is the first report of bullous hemorrhagic dermatosis induced by enoxaparin with underlying bullous pemphigoid in Korea.
Figures and Tables
Fig. 1
Bullous hemorrhagic lesions. (A) Tense, hemorrhagic bulla on the right forearm. (B) Ruptured hemorrhagic bulla on the right forearm. (C) Regressed skin lesion at 4 weeks after the development.

Fig. 2
Representative figures of histopathological examination. (A) Intradermal vesicle formation with filled red blood cells (×4). (B) Mildly perivascular lymphocytic and a few eosinophilic infiltration in periphery of vesicle. No evidences of vasculitis or intravascular thrombus (×20). (C) C3 deposition of dermo-epidemal junction in immunofluorescence (×10).
References
3. Gouveia AI, Lopes L, Soares-Almeida L, Filipe P. Bullous hemorrhagic dermatosis induced by enoxaparin. Cutan Ocul Toxicol. 2016; 35:160–162.

4. Roux J, Duong TA, Ingen-Housz-Oro S, Valeyrie-Allanore L, Ortonne N, Chosidow O, Wolkenstein P. Heparin-induced hemorrhagic blisters. Eur J Dermatol. 2013; 23:105–107.

5. Thuillier D, Chaby G, Dadban A, Dascotte E, Miquel-Christophe O, Andrejak M, Chatelain D, Lok C. Low-molecular-weight heparin-induced bullous haemorrhagic dermatosis associated with cell-mediated hypersensitivity. Ann Dermatol Venereol. 2009; 136:705–708.
6. Perrinaud A, Jacobi D, Machet MC, Grodet C, Gruel Y, Machet L. Bullous hemorrhagic dermatosis occurring at sites distant from subcutaneous injections of heparin: three cases. J Am Acad Dermatol. 2006; 54:2 Suppl. S5–S7.

7. Naveen KN, Rai V. Bullous hemorrhagic dermatosis: a case report. Indian J Dermatol. 2014; 59:423.

8. Gonzales UP, Scott GA, Briones AJ, Pentland AP. Remote hemorrhagic bullae occurring in a patient treated with subcutaneous heparin. Arch Dermatol. 2009; 145:604–605.

9. Peña ZG, Suszko JW, Morrison LH. Hemorrhagic bullae in a 73-year-old man. Bullous hemorrhagic dermatosis related to enoxaparin use. JAMA Dermatol. 2013; 149:871–872.
10. Govind B, Gnass E, Merli G, Eraso L. Hemorrhagic bullous dermatosis: a rare heparin-induced cutaneous manifestation. Hosp Pract (1995). 2016; 44:103–107.

11. Villanueva CA, Nájera L, Espinosa P, Borbujo J. Bullous hemorrhagic dermatosis at distant sites: a report of 2 new cases due to enoxaparin injection and a review of the literature. Actas Dermosifiliogr. 2012; 103:816–819.

12. Deser SB, Demirag MK. Low molecular weight heparin (LMWH)-induced bullous hemorrhagic dermatosis. J Card Surg. 2015; 30:568–569.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download