Abstract
Recent studies suggest that vitamin D is related to allergic rhinitis (AR). In this review, we first discuss the physiology and metabolism of vitamin D, then we review the function of vitamin D in the immune system, and above all, we highlight the current research regarding the role of vitamin D in AR. Finally, we find that there are both experimental and clinical studies showing that vitamin D is associated with AR, although the results are not consistent and even conflicting. Evidences from those clinical studies show a slightly tendency that serum vitamin D level might be inversely associated with the risk of AR. Meanwhile, it seems that gender and age may influence the relationship between vitamin D and AR. However, because of the heterogeneity in defining AR, differences in study design and so on, all these findings need to be confirmed by further studies. Additional clinical studies as well as experimental research are needed to better understand how vitamin D influences AR.
Allergic rhinitis (AR) is a symptomatic disorder of the nose induced after allergen exposure through IgE-mediated inflammation of the membranes lining the nose. AR is a global health problem that causes major illness and disability worldwide, affecting patients' social life, sleep, and their school and work performance. A 2-step, cross-sectional, population-based survey in Europe revealed that the prevalence of clinically confirmable AR ranged from 16.9%–28.5% in subjects studied [1]. Additionally, the self-reported prevalence of AR in eleven major cities in mainland China ranged from 8.7%–24.1% [2]. Therefore, AR has become a large burden in society worldwide. Although clinical practice guidelines for the management of AR that have been developed over the past decade have improved the care of patients with AR [34], the exact pathogenesis of AR remains unclear. It is believed that both environmental factors and genetic susceptibility play a role in the etiology of AR.
Recently, many studies have reported that vitamin D may be associated with the development of AR. In this review, we aim to discuss the physiology and metabolism of vitamin D, the function of vitamin D in the immune system, and above all, we highlight the current research regarding the role of vitamin D in AR.
Vitamin D has long been known to be an essential nutrient for the human body, particularly with regard to the absorption of dietary calcium and phosphate [5]. Technically, vitamin D is not a true vitamin; it belongs to the family of steroid hormones. Its nuclear hormone receptor, vitamin D receptor (VDR), is expressed in at least seventeen tissues or cells [67].
Vitamin D has 2 major forms, cholecalciferol (vitamin D3) and ergocalciferol (vitamin D2). Both forms of vitamin D (D2 and D3) can be found in foods or supplements; however, only vitamin D3 is produced in skin [8], and it is the only naturally occurring form of vitamin D in humans and other animals [6]. Human vitamin D endocrine system includes 3 forms of vitamin D [56], namely vitamin D3, calcidiol (25(OH)D3), and calcitriol (1,25(OH)2D3). Vitamin D3 is the naturally occurring form of vitamin D, derived from either dietary sources or formed from 7-dehydrocholesterol (7-DHC or provitamin D3) by the skin. 25(OH)D3 is a prehormone in the blood that is made directly from vitamin D3, and it is also what is directly tested to measure vitamin D3 in the blood (To clarify, 25(OH)D levels usually contain both the vitamin D2 and D3 forms). 25(OH)D3 is an active form of vitamin D3. 1,25(OH)2D3, which is made from 25(OH)D3, is the hormone form of vitamin D3 and the most biologically active metabolite of vitamin D3.
Upon exposure to ultraviolet B radiation (wavelengths of 290–315 nm), 7-DHC transforms into vitamin D3, which enters the circulation and binds vitamin D-binding protein. In the classic vitamin D3 pathway, vitamin D3 then undergoes hydroxylation in the 25-position by vitamin D-25-hydroxylase (25-OHase) in the liver to form 25(OH)D3 and in the 1-position by 25-hydroxyvitamin D-1-alpha-hydroxylase (1α-OHase) in the kidney (functioning as an endocrine gland) to form 1,25(OH)2D3, which is the form that promotes intestinal absorption of calcium and phosphate (Fig. 1).
In the nonclassic vitamin D3 pathway (Fig. 1), other cells that also possess the 1α-OHase protein, including cells of the immune system, produce the hormonal form of vitamin D3 from 25(OH)D3, and they respond in an autocrine or paracrine fashion [89]. This pathway differs from the classic one in that the extrarenal 1α-OHase is not regulated by parathyroid hormone and that there is no negative feedback of local 1,25(OH)2D3 by circulating 1,25(OH)2D3. 1,25(OH)2D3 enters target cells from the circulation and binds to VDR in the cytoplasm. After binding, they enter the nucleus and heterodimerize with the retinoid X receptor (RXR). The 1,25(OH)2D3-RXR-VDR complex then binds to vitamin D response elements located on the DNA and initiates biological responses through regulation of gene transcription and activation of a variety of signal transduction pathways at or near the plasma membrane [568].
The hormonal form of vitamin D3 has been recognized as an immunoregulatory hormone for 30 years [10]. Experimental studies have demonstrated that 1,25(OH)2D3 affects a wide range of immune cells and cytokines and is associated with many immune diseases.
Previous reports have shown that T cells, B cells, dendritic cells (DCs), monocytes, and macrophages are all influenced by the regulation of 1,25(OH)2D3 [7811121314151617181920212223]. 1,25(OH)2D3 inhibits T-cell proliferation [11, 12]; facilitates induction of Foxp3+ T regulatory (Treg) cells [13]; suppresses the differentiation, maintenance, bioactivity, and transcription of Th17 cells [1415]; and induces the switch from Th1 to Th2 by decreasing the Th1 response via regulation of antigen presenting cells and enhancing Th2 cell development [1617]. Additionally, 1,25(OH)2D3 inhibits the proliferation and induces apoptosis of activated B cells, and it inhibits plasma cell differentiation and immunoglobulin secretion [18], including IgE secretion [19]. The effect of 1,25(OH)2D3 on DCs is more complicated. 1,25(OH)2D3 interferes with the differentiation of DCs from human monocytes, and DCs differentiated in the presence of 1,25(OH)2D3 showed inhibited immunostimulatory capacity [20]. Thus, 1,25(OH)2D3 leads to the development of DCs with tolerogenic properties, as immature DCs promote T-cell tolerance; whereas, mature DCs activate naïve T cells [8]. In monocytes, 1,25(OH)2D3 increases the effectiveness of the antimicrobial activity of freshly isolated monocytes but induces monocytes in more mature stages of differentiation to exert anti-inflammatory effects [21]. Finally, 1,25(OH)2D3 decreases macrophage inflammation and T-cell stimulation [22].
What's more, VDR, 25-OHase, and 1α-OHase are required for the activation and direct function of 1,25(OH)2D3, and recent studies have detected the expression and production of all of these proteins in several immune cells, including lymphocytes, monocytes, macrophages, and DCs [72324], also suggesting that 1,25(OH)2D3 plays a role in the regulation of these immune cells.
In addition to its effects on immune cells, 1,25(OH)2D3 also modulates a variety of cytokines, some of which may play a key role in the pathogenesis of many autoimmune diseases. Although conflicting data exist, most studies suggest that Th1-related cytokines (e.g., interferon-γ, interleukin [IL]-2, tumor necrosis factor-α, IL-12) [252627] are generally suppressed by exposure to 1,25(OH)2D3, while Th2-related cytokines (e.g., IL-4, IL-10) [16] are upregulated by 1,25(OH)2D3.
An increasing number of epidemiological studies have linked vitamin D levels with allergic disorders, especially asthma.
In a cross-sectional study of 170 Turkish children (85 asthmatic and 85 healthy patients), the mean 25(OH)D3 level in the asthma group was significantly different than that of the healthy control group, and a decrease in 25(OH)D3 levels increased the severity of asthma and decreased the frequency of controlled asthma [28]. The result was consistent with another study that consisted of 70 Saudi children aged 4–18 years, which showed that hypovitaminosis D is highly prevalent among asthmatic children and is positively related to asthma severity as compared with healthy control children [29]. In another large adult population-based cohort study conducted in Israel, although no significant association between 25(OH)D status and physician-diagnosed asthma was found, there was a strong association with asthma exacerbations [30]. Lately, Tachimoto et al. [31] reported a randomized, double-blind, placebo-controlled trial, which compared vitamin D3 supplements (800 IU/day) with placebo for 2 months in schoolchildren with asthma, and they found that asthma control was significantly more improved in the vitamin D group compared with the placebo group. However, in another latest double-blind, randomized, placebo-controlled study, Kerley et al. [32] assessed the role of vitamin D3 supplementation (2,000 IU/day, 15 weeks) in childhood asthma in Ireland, and they found that except for a significant decreased school days missed, no other advantageous changes in asthma parameters compared to placebo. Results from the above studies were basically consistent with that of the systematic review conducted by Cassim et al. [33] recently. They searched articles that measured the association between serum vitamin D and asthma incidence, prevalence, severity and exacerbations in the PubMed database. Evidence suggested that higher serum levels of 25(OH)D were associated with a reduced risk of asthma exacerbations, but there was little evidence to suggest an association with asthma incidence, prevalence or severity.
Studies were also reported in the situation of other allergic disorders, and even some autoimmune diseases. In a late randomized, double-blind, placebo-controlled parallel-group trial, the authors found that vitamin D supplementation during pregnancy and infancy reduced the proportion of children sensitized to mites at age 18 months [34]. A study from Heimbeck et al. [35] suggested that low serum 25(OH)D level was inversely associated with prevalence of eczema in German children and adolescents. In another study which examined the associations between early-life vitamin D levels and the development of allergy-related outcomes (eczema, skin prick tests, increased allergen-specific IgE level, and doctor's diagnosis of asthma), prenatal 25(OH)D levels were also inversely associated with eczema [36]. As for autoimmune diseases, vitamin D deficiency was associated with type 1 diabetes mellitus (T1DM) in Egyptian children in a case-control study [37], and serum 25(OH)D levels were deficient in 75% of the T1DM patients, while the mean levels of vitamin D were significantly lower in patients as compared to their controls. Another case-control study of Indian patients revealed that plasma 25(OH)D3 levels were inversely correlated with systemic lupus erythematosus disease activity index scores [38]. Additionally, in a cross-sectional study of 66 newly diagnosed rheumatoid arthritis (RA) patients in Iran, patients with more active RA had lower serum 25(OH)D levels [39]. Literatures above suggested that lower serum levels of 25(OH)D/25(OH)D3 was a risk factor to the prevalence, severity and exacerbations of those diseases.
Given the important role of vitamin D in the immune system, the potential relationship between vitamin D and AR has received much interest in recent years (Fig. 2).
It is generally agreed that a shift from a Th1 to Th2 phenotype in the proliferation of CD4+ T cells contributes to the pathogenesis of AR; however, the exact mechanism is still under investigation. Recent studies indicate that Th17 and Treg cells are important in the disease course of AR [40]. As previously summarized, vitamin D inhibits the proliferation of T cells; induces a switch from Th1 to Th2 by enhancing the development of Th2 cells; facilitates the induction of Foxp3+ Treg cells; and suppresses the differentiation, maintenance, bioactivity, and transcription of Th17 cells. These data indicate there is a relationship between vitamin D and AR morbidity.
As with epidemiological and clinical studies that have found an association between vitamin D levels and previously mentioned allergic disorders/autoimmune diseases, recent reports suggest a relationship between vitamin D levels and the incidence of AR in different ethnic groups, although different studies may have some difference in defining AR. Hyppönen et al. [41] investigated the associations between infant vitamin D supplementation and allergic conditions in adulthood using a cohort of subjects born in 1966 in Finland. They found that the prevalence of AR (They defined AR when participants reported allergic cold—related to contact with animals or pollen, e.g., hay fever—during the past 12 months.) at age 31 years was higher in participants who had received vitamin D supplementation regularly during the first year of their life compared to those who had not received supplementation. In another subsequent study, Wjst and Hyppönen [42] analyzed the association between serum 25(OH)D3 levels and AR prevalence (AR was defined by the question “Did a doctor ever tell you had hay fever?”) in adults using the Third National Health and Nutrition Examination Survey (NHANES III) study in Germany, and found that AR prevalence increased with levels of 25(OH)D3 in all subgroups (divided by 25(OH)D3 quartile level) and following adjustments for sex, geographical region, and month of examination. Findings from these 2 reports suggested that vitamin D supplementation in infancy or high levels of 25(OH)D3 was positively related to AR prevalence in adults.
Nonetheless, it seems more contradictory results have been
reported as well. For example, Bunyavanich et al. [43] reported their study of 1,248 mother-child pairs from a US prebirth cohort unselected for any disease, and they found that each 100 IU/day of food-based vitamin D intake during the first and second trimesters was associated with 21% and 20% reduced odds of ever AR at school age (Ever AR at school age was defined as positive if a mother answered yes to “Have you ever been told by a health care professional, such as a doctor, physician assistant or nurse practitioner, that your child has hay fever, seasonal allergies or AR (runny nose due to allergies)?” at the school-age interview.), respectively. But there were no associations between maternal supplemental vitamin D intake or serum 25(OH)D levels at any time point with ever AR. Dogru and Suleyman [44] compared the serum 25(OH)D3 levels in children with AR (AR was classified according to the Allergic Rhinitis and its Impact on Asthma [ARIA] 2008 guidelines.) or nonallergic rhinitis (NAR) with the control group, and they found the mean serum 25(OH)D3 levels of the children both with AR and NAR were lower than control group. But they did not find any relationship between 25(OH)D3 levels and the severity and duration of AR. A cross-sectional study conducted in Qatar found that 25(OH)D deficiency significantly correlated with AR (No specific description of AR definition was found in the article.) in children [45]. Another study, using data from the fourth annual Korean National Health and Nutrition Examination Survey (2009), revealed that the mean 25(OH)D level of the AR (Participants were determined as having AR when they answered ‘yes’ to the survey item ‘AR diagnosed by a doctor.’) group was lower than that of the non-AR group, even after adjusting for body mass index (BMI), smoking status, age, sex, sun exposure, income quartile, exercise, and body fat percentage [46]. In another study in Iran, Arshi et al. [47] measured the 25(OH)D levels in patients with AR (AR patients were diagnosed clinically using ARIA 2008 criteria during a medical visit.) and compared the results with the general population (no control group), they found prevalence of severe 25(OH)D deficiency was significantly higher in AR patients than the normal population. Also women with AR had lower 25(OH)D levels. Interestingly, Mai et al. [48] recently reported results from the HUNT study (the Nord-Trøndelag Health Study), which found that vitamin D appeared to play different roles in the development of AR (AR was self-reported according to the questions: “Do you have or have you had allergic rhinitis or hay fever?”) among men and women in Norway. In this Norwegian adult population who reported no AR at baseline, they found that lower serum 25(OH)D levels were associated with an increased risk of AR among men but a reduced risk of AR among women, especially premenopausal women.
In addition, there are also studies that have found no associations between vitamin D intake [49] in midpregnancy and child AR (AR was self-reported according to questionnaires.), or between serum 25(OH)D levels [50] and AR (AR was defined by the question: “Have you been diagnosed with AR by a doctor?”).
We can see from the above studies that only 2 studies [4447] defined AR according to ARIA, and both of them reported negative association between vitamin D levels and AR. Almost all the other studies were self-reported AR by questions or questionnaires [41424346484950] (No specific description of AR definition was found in one study [45].), among them, three articles reported negative association between vitamin D levels and AR, while 2 reported positive association and another 2 reported no association between vitamin D levels and AR. Actually, not only there are differences in defining AR, some of the studies are interventional ones that concern vitamin D supplementation/intake during infancy [41] or mother's pregnancy [4349], while some others are observational ones [42444546474850] which only detect the association between serum vitamin D level and AR. Taken the current 3 interventional studies [414349] together, we find that infant vitamin D supplementation may be associated with risk of adulthood AR [41], but maternal food-based vitamin D intake rather than vitamin D supplementation may reduce the risk of childhood AR [4349]. These results are too obscure to draw any conclusions, but we figure that maternal vitamin D intake (food-based) might reduce the risk of childhood AR, while infant and maternal vitamin D supplementation might do no good to reduce AR (in adulthood and in childhood, respectively). Likewise, it is also difficult to draw any simple conclusions in the situation of current observational studies.
Besides of the heterogeneity in definition of AR and vitamin D supplementation/intake or not, we consider that there may be some other reasons for the controversy of current studies regarding the relationship between AR and vitamin D, and they are as follows: (1) Experimental data support a link between vitamin D and AR; however, the exact mechanism of how vitamin D influences the pathogenesis of AR is unclear. Vitamin D inhibits the proliferation of T cells, facilitates the induction of Foxp3+ Treg cells, and suppresses the differentiation, maintenance, bioactivity, and transcription of Th17 cells, suggesting that vitamin D may decrease AR-related inflammation. However, vitamin D also shifts the Th1/Th2 balance towards Th2, which suggests vitamin D may lead to AR (Fig. 2). Further research is needed to help determine which of these effects is most important, as well as whether the timing of these mechanisms influences the development of AR. (2) Conflicting conclusions exist regarding the relationship between vitamin D levels and the risk of AR in women and men [48] as well as in adults and children [4244 45] (Fig. 2). It is possible that gender and age may influence the relationship between vitamin D and AR. (3) Different study designs can lead to different conclusions. Results from current literatures were obtained from cohort studies, cross-sectional studies and case-control studies. Meanwhile, more studies detected 25(OH)D levels while the others tested 25(OH)D3 levels by using different methods, and some studies focused on vitamin D intake while some researched vitamin D deficiency.
Despite the conflicts, we consider evidences from present clinical trials suggest a slightly tendency that serum vitamin D level might be inversely associated with the risk of AR versus the opposite conclusion (Fig. 2). Nonetheless, more well-designed studies are needed to complement the current ones to further investigate the relationship between vitamin D levels and AR, and the potential influence of other factors on this relationship, since AR is a complex disease with a varying course and severity, which often occurs in conjunction with other immune diseases, and vitamin D levels are influenced by numerous factors, such as sunshine, diet, BMI, accompanying diseases, or skin color.
In addition to environmental influences, genetic susceptibility is also a determining factor in the etiology of AR, and there may be a genetic role in the effect of vitamin D on AR. There is supporting evidence for this hypothesis: (1) Vitamin D exerts its function through the vitamin D endocrine system, which includes VDR, 25-OHase, and 1α-OHase. Genetic differences may affect how vitamin D and the vitamin D endocrine system influence the development and severity of AR in different individuals. (2) Genes of some members of the vitamin D endocrine system map to the susceptibility loci for allergic diseases according to genome-wide linkage analysis [51]. (3) Gene polymorphisms of some members of the system are associated with susceptibility to other immune diseases such as asthma [52], which has a similar pathogenesis as AR. In fact, in our recent study, we have found that age and sex may have an impact on the association of 3 single nucleotide polymorphisms (rs2228570, rs731236, and rs2060793) in genes of the vitamin D pathway with the risk of mite-sensitized persistent AR in a Chinese population [53]. We believe more genetic studies investigating the association between vitamin D and AR are also needed and may shed new light on the etiology of AR.
Vitamin D is a steroid rather than a vitamin with a complicated endocrine system that allows the active form of vitamin D to function properly in human body. Vitamin D is a regulatory factor in the human immune system, and many immune diseases are related to vitamin D levels. Both experimental and clinical studies have shown that vitamin D is associated with AR, although the results are not consistent and even conflicting. Evidences from those clinical studies show a slightly tendency that serum vitamin D level might be inversely associated with the risk of AR. Meanwhile, it seems that gender and age may influence the relationship between vitamin D and AR. All these findings need to be confirmed by more studies. Genetically, the vitamin D endocrine system may have an association with AR, which may also influence the etiology of AR. Additional studies are needed to better understand how vitamin D influences AR.
Figures and Tables
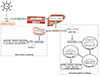 | Fig. 1Physiology and metabolism of vitamin D. Upon exposure to ultraviolet B radiation (UVB, wavelengths of 290–315 nm), 7-dehydrocholesterol (7-DHC) transforms into vitamin D3, which enters the circulation and binds vitamin D-binding protein (VDBP). In the classic vitamin D3 pathway, vitamin D3 then undergoes hydroxylation in the 25-position by vitamin D-25-hydroxylase (25-OHase) in the liver to form 25(OH)D3 and in the 1-position by 25-hydroxyvitamin D-1-alpha-hydroxylase (1α-OHase) in the kidney (functioning as an endocrine gland) to form 1,25(OH)2D3. In the nonclassic vitamin D3 pathway, other cells that also possess the 1α-OHase protein produce 1,25(OH)2D3. 1,25(OH)2D3 enters target cells from the circulation and binds to vitamin D receptor (VDR) in the cytoplasm. After binding, they enter the nucleus and heterodimerize with the retinoid X receptor (RXR). The 1,25(OH)2D3-RXR-VDR complex then binds to vitamin D response elements located on the DNA and initiates biological responses through regulation of gene transcription and activation of a variety of signal transduction pathways. PTH, parathyroid hormone. |
ACKNOWLEDGEMENTS
This work was supported by grants from the National Natural Science Foundation (81300834), the Priority Academic Program Development of Jiangsu Higher Education Institutions (JX10231801), and the Health Promotion Project of Jiangsu Province (XK200719, RC2007065 and RC2011071), the People's Republic of China. We thank Professor De Yun Wang at the Department of Otolaryngology, Yong Loo Lin School of Medicine, National University of Singapore for kind advice in the manuscript preparation.
References
1. Bauchau V, Durham SR. Prevalence and rate of diagnosis of allergic rhinitis in Europe. Eur Respir J. 2004; 24:758–764.

2. Zhang L, Han D, Huang D, Wu Y, Dong Z, Xu G, Kong W, Bachert C. Prevalence of self-reported allergic rhinitis in eleven major cities in china. Int Arch Allergy Immunol. 2009; 149:47–57.

3. Cheng L, Chen YZ. ARIA expert panel. Allergic Rhinitis and its Impact on Asthma (ARIA) achievements in 10 years and future needs. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2012; 47:619–622.
4. Bousquet J, Schünemann HJ, Samolinski B, Demoly P, Baena-Cagnani CE, Bachert C, Bonini S, Boulet LP, Bousquet PJ, Brozek JL, Canonica GW, Casale TB, Cruz AA, Fokkens WJ, Fonseca JA, van Wijk RG, Grouse L, Haahtela T, Khaltaev N, Kuna P, Lockey RF, Lodrup Carlsen KC, Mullol J, Naclerio R, O'Hehir RE, Ohta K, Palkonen S, Papadopoulos NG, Passalacqua G, Pawankar R, Price D, Ryan D, Simons FE, Togias A, Williams D, Yorgancioglu A, Yusuf OM, Aberer W, Adachi M, Agache I, Aït-Khaled N, Akdis CA, Andrianarisoa A, Annesi-Maesano I, Ansotegui IJ, Baiardini I, Bateman ED, Bedbrook A, Beghé B, Beji M, Bel EH, Ben Kheder A, Bennoor KS, Bergmann KC, Berrissoul F, Bieber T, Bindslev Jensen C, Blaiss MS, Boner AL, Bouchard J, Braido F, Brightling CE, Bush A, Caballero F, Calderon MA, Calvo MA, Camargos PA, Caraballo LR, Carlsen KH, Carr W, Cepeda AM, Cesario A, Chavannes NH, Chen YZ, Chiriac AM, Chivato Pérez T, Chkhartishvili E, Ciprandi G, Costa DJ, Cox L, Custovic A, Dahl R, Darsow U, De Blay F, Deleanu D, Denburg JA, Devillier P, Didi T, Dokic D, Dolen WK, Douagui H, Dubakiene R, Durham SR, Dykewicz MS, El-Gamal Y, El-Meziane A, Emuzyte R, Fiocchi A, Fletcher M, Fukuda T, Gamkrelidze A, Gereda JE, González Diaz S, Gotua M, Guzmán MA, Hellings PW, Hellquist-Dahl B, Horak F, Hourihane JO, Howarth P, Humbert M, Ivancevich JC, Jackson C, Just J, Kalayci O, Kaliner MA, Kalyoncu AF, Keil T, Keith PK, Khayat G, Kim YY, Koffi N'goran B, Koppelman GH, Kowalski ML, Kull I, Kvedariene V, Larenas-Linnemann D, Le LT, Lemière C, Li J, Lieberman P, Lipworth B, Mahboub B, Makela MJ, Martin F, Marshall GD, Martinez FD, Masjedi MR, Maurer M, Mavale-Manuel S, Mazon A, Melen E, Meltzer EO, Mendez NH, Merk H, Mihaltan F, Mohammad Y, Morais-Almeida M, Muraro A, Nafti S, Namazova-Baranova L, Nekam K, Neou A, Niggemann B, Nizankowska-Mogilnicka E, Nyembue TD, Okamoto Y, Okubo K, Orru MP, Ouedraogo S, Ozdemir C, Panzner P, Pali-Schöll I, Park HS, Pigearias B, Pohl W, Popov TA, Postma DS, Potter P, Rabe KF, Ratomaharo J, Reitamo S, Ring J, Roberts R, Rogala B, Romano A, Roman Rodriguez M, Rosado-Pinto J, Rosenwasser L, Rottem M, Sanchez-Borges M, Scadding GK, Schmid-Grendelmeier P, Sheikh A, Sisul JC, Solé D, Sooronbaev T, Spicak V, Spranger O, Stein RT, Stoloff SW, Sunyer J, Szczeklik A, Todo-Bom A, Toskala E, Tremblay Y, Valenta R, Valero AL, Valeyre D, Valiulis A, Valovirta E, Van Cauwenberge P, Vandenplas O, van Weel C, Vichyanond P, Viegi G, Wang DY, Wickman M, Wöhrl S, Wright J, Yawn BP, Yiallouros PK, Zar HJ, Zernotti ME, Zhong N, Zidarn M, Zuberbier T, Burney PG, Johnston SL, Warner JO. World Health Organization Collaborating Center for Asthma and Rhinitis. Allergic Rhinitis and its Impact on Asthma (ARIA): achievements in 10 years and future needs. J Allergy Clin Immunol. 2012; 130:1049–1062.

5. Akbar NA, Zacharek MA. Vitamin D: immunomodulation of asthma, allergic rhinitis, and chronic rhinosinusitis. Curr Opin Otolaryngol Head Neck Surg. 2011; 19:224–228.

6. Norman AW. From vitamin D to hormone D: fundamentals of the vitamin D endocrine system essential for good health. Am J Clin Nutr. 2008; 88:491S–499S.

7. Wang Y, Zhu J, DeLuca HF. Where is the vitamin D receptor? Arch Biochem Biophys. 2012; 523:123–133.

8. Kamen DL, Tangpricha V. Vitamin D and molecular actions on the immune system: modulation of innate and autoimmunity. J Mol Med (Berl). 2010; 88:441–450.

9. Fritsche J, Mondal K, Ehrnsperger A, Andreesen R, Kreutz M. Regulation of 25-hydroxyvitamin D3-1 alpha-hydroxylase and production of 1 alpha,25-dihydroxyvitamin D3 by human dendritic cells. Blood. 2003; 102:3314–3316.
10. Tsoukas CD, Provvedini DM, Manolagas SC. 1,25-dihydroxyvitamin D3: a novel immunoregulatory hormone. Science. 1984; 224:1438–1440.
11. Rigby WF, Stacy T, Fanger MW. Inhibition of T lymphocyte mitogenesis by 1,25-dihydroxyvitamin D3 (calcitriol). J Clin Invest. 1984; 74:1451–1455.

12. Cantorna MT, Waddell A. The vitamin D receptor turns off chronically activated T cells. Ann N Y Acad Sci. 2014; 1317:70–75.
13. Urry Z, Chambers ES, Xystrakis E, Dimeloe S, Richards DF, Gabryšová L, Christensen J, Gupta A, Saglani S, Bush A, O'Garra A, Brown Z, Hawrylowicz CM. The role of 1α,25-dihydroxyvitamin D3 and cytokines in the promotion of distinct Foxp3+ and IL-10+ CD4+ T cells. Eur J Immunol. 2012; 42:2697–2708.
14. Zhang H, Shih DQ, Zhang X. Mechanisms underlying effects of 1,25-Dihydroxyvitamin D3 on the Th17 cells. Eur J Microbiol Immunol (Bp). 2013; 3:237–240.
15. Hamzaoui A, Berraïes A, Hamdi B, Kaabachi W, Ammar J, Hamzaoui K. Vitamin D reduces the differentiation and expansion of Th17 cells in young asthmatic children. Immunobiology. 2014; 219:873–879.

16. Boonstra A, Barrat FJ, Crain C, Heath VL, Savelkoul HF, O'Garra A. 1alpha,25-Dihydroxyvitamin d3 has a direct effect on naive CD4(+) T cells to enhance the development of Th2 cells. J Immunol. 2001; 167:4974–4980.
17. Vasiliou JE, Lui S, Walker SA, Chohan V, Xystrakis E, Bush A, Hawrylowicz CM, Saglani S, Lloyd CM. Vitamin D deficiency induces Th2 skewing and eosinophilia in neonatal allergic airways disease. Allergy. 2014; 69:1380–1389.
18. Chen S, Sims GP, Chen XX, Gu YY, Chen S, Lipsky PE. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J Immunol. 2007; 179:1634–1647.
19. Heine G, Anton K, Henz BM, Worm M. 1alpha,25-dihydroxyvitamin D3 inhibits anti-CD40 plus IL-4-mediated IgE production in vitro. Eur J Immunol. 2002; 32:3395–3404.
20. Piemonti L, Monti P, Sironi M, Fraticelli P, Leone BE, Dal Cin E, Allavena P, Di Carlo V. Vitamin D3 affects differentiation, maturation, and function of human monocyte-derived dendritic cells. J Immunol. 2000; 164:4443–4451.
21. Di Rosa M, Malaguarnera G, De Gregorio C, Palumbo M, Nunnari G, Malaguarnera L. Immuno-modulatory effects of vitamin D3 in human monocyte and macrophages. Cell Immunol. 2012; 280:36–43.

22. Korf H, Wenes M, Stijlemans B, Takiishi T, Robert S, Miani M, Eizirik DL, Gysemans C, Mathieu C. 1,25-Dihydroxyvitamin D3 curtails the inflammatory and T cell stimulatory capacity of macrophages through an IL-10-dependent mechanism. Immunobiology. 2012; 217:1292–1300.

23. Morán-Auth Y, Penna-Martinez M, Shoghi F, Ramos-Lopez E, Badenhoop K. Vitamin D status and gene transcription in immune cells. J Steroid Biochem Mol Biol. 2013; 136:83–85.

24. Adorini L, Penna G, Giarratana N, Roncari A, Amuchastegui S, Daniel KC, Uskokovic M. Dendritic cells as key targets for immunomodulation by Vitamin D receptor ligands. J Steroid Biochem Mol Biol. 2004; 89-90:437–441.

25. D'Ambrosio D, Cippitelli M, Cocciolo MG, Mazzeo D, Di Lucia P, Lang R, Sinigaglia F, Panina-Bordignon P. Inhibition of IL-12 production by 1,25-dihydroxyvitamin D3. Involvement of NF-kappaB downregulation in transcriptional repression of the p40 gene. J Clin Invest. 1998; 101:252–262.
26. Cippitelli M, Santoni A. Vitamin D3: a transcriptional modulator of the interferon-gamma gene. Eur J Immunol. 1998; 28:3017–3030.
27. Almerighi C, Sinistro A, Cavazza A, Ciaprini C, Rocchi G, Bergamini A. 1Alpha,25-dihydroxyvitamin D3 inhibits CD40L-induced pro-inflammatory and immunomodulatory activity in human monocytes. Cytokine. 2009; 45:190–197.
28. Uysalol M, Mutlu LC, Saracoglu GV, Karasu E, Guzel S, Kayaoglu S, Uzel N. Childhood asthma and vitamin D deficiency in Turkey: is there cause and effect relationship between them? Ital J Pediatr. 2013; 39:78.

29. Aldubi HM, Alissa EM, Kamfar HZ, Gaber O, Marzouki ZM. Bronchial asthma and hypovitaminosis D in Saudi children. Asia Pac Allergy. 2015; 5:103–113.

30. Confino-Cohen R, Brufman I, Goldberg A, Feldman BS. Vitamin D, asthma prevalence and asthma exacerbations: a large adult population-based study. Allergy. 2014; 69:1673–1680.

31. Tachimoto H, Mezawa H, Segawa T, Akiyama N, Ida H, Urashima M. Improved control of childhood asthma with low-dose, short-term vitamin D supplementation: a randomized, double-blind, placebo-controlled trial. Allergy. 2016; 71:1001–1009.

32. Kerley CP, Hutchinson K, Cormican L, Faul J, Greally P, Coghlan D, Elnazir B. Vitamin D3 for uncontrolled childhood asthma: a pilot study. Pediatr Allergy Immunol. 2016; 27:404–412.
33. Cassim R, Russell MA, Lodge CJ, Lowe AJ, Koplin JJ, Dharmage SC. The role of circulating 25 hydroxyvitamin D in asthma: a systematic review. Allergy. 2015; 70:339–354.

34. Grant CC, Crane J, Mitchell EA, Sinclair J, Stewart A, Milne T, Knight J, Gilchrist C, Camargo CA Jr. Vitamin D supplementation during pregnancy and infancy reduces aeroallergen sensitization: a randomized controlled trial. Allergy. 2016; 71:1325–1334.

35. Heimbeck I, Wjst M, Apfelbacher CJ. Low vitamin D serum level is inversely associated with eczema in children and adolescents in Germany. Allergy. 2013; 68:906–910.

36. Wegienka G, Havstad S, Zoratti EM, Kim H, Ownby DR, Johnson CC. Association between vitamin D levels and allergy-related outcomes vary by race and other factors. J Allergy Clin Immunol. 2015; 136:1309–1314.

37. Abd-Allah SH, Pasha HF, Hagrass HA, Alghobashy AA. Vitamin D status and vitamin D receptor gene polymorphisms and susceptibility to type 1 diabetes in Egyptian children. Gene. 2014; 536:430–434.

38. Mandal M, Tripathy R, Panda AK, Pattanaik SS, Dakua S, Pradhan AK, Chakraborty S, Ravindran B, Das BK. Vitamin D levels in Indian systemic lupus erythematosus patients: association with disease activity index and interferon alpha. Arthritis Res Ther. 2014; 16:R49.

39. Zakeri Z, Sandoughi M, Mashhadi MA, Raeesi V, Shahbakhsh S. Serum vitamin D level and disease activity in patients with recent onset rheumatoid arthritis. Int J Rheum Dis. 2016; 19:343–347.

40. Osguthorpe JD. Pathophysiology of and potential new therapies for allergic rhinitis. Int Forum Allergy Rhinol. 2013; 3:384–392.

41. Hyppönen E, Sovio U, Wjst M, Patel S, Pekkanen J, Hartikainen AL, Järvelinb MR. Infant vitamin d supplementation and allergic conditions in adulthood: northern Finland birth cohort 1966. Ann N Y Acad Sci. 2004; 1037:84–95.

43. Bunyavanich S, Rifas-Shiman SL, Platts-Mills TA, Workman L, Sordillo JE, Camargo CA Jr, Gillman MW, Gold DR, Litonjua AA. Prenatal, perinatal, and childhood vitamin D exposure and their association with childhood allergic rhinitis and allergic sensitization. J Allergy Clin Immunol. 2016; 137:1063–1070.e1-2.

44. Dogru M, Suleyman A. Serum 25-hydroxyvitamin D3 levels in children with allergic or nonallergic rhinitis. Int J Pediatr Otorhinolaryngol. 2016; 80:39–42.

45. Bener A, Ehlayel MS, Bener HZ, Hamid Q. The impact of Vitamin D deficiency on asthma, allergic rhinitis and wheezing in children: An emerging public health problem. J Family Community Med. 2014; 21:154–161.

46. Jung JW, Kim JY, Cho SH, Choi BW, Min KU, Kang HR. Allergic rhinitis and serum 25-hydroxyvitamin D level in Korean adults. Ann Allergy Asthma Immunol. 2013; 111:352–357.

47. Arshi S, Ghalehbaghi B, Kamrava SK, Aminlou M. Vitamin D serum levels in allergic rhinitis: any difference from normal population? Asia Pac Allergy. 2012; 2:45–48.

48. Mai XM, Chen Y, Camargo CA Jr, Langhammer A. Serum 25-hydroxyvitamin D levels and self-reported allergic rhinitis in Norwegian adults: The HUNT Study. Allergy. 2014; 69:488–493.
49. Maslova E, Hansen S, Jensen CB, Thorne-Lyman AL, Strøm M, Olsen SF. Vitamin D intake in mid-pregnancy and child allergic disease: a prospective study in 44,825 Danish mother-child pairs. BMC Pregnancy Childbirth. 2013; 13:199.

50. Cheng HM, Kim S, Park GH, Chang SE, Bang S, Won CH, Lee MW, Choi JH, Moon KC. Low vitamin D levels are associated with atopic dermatitis, but not allergic rhinitis, asthma, or IgE sensitization, in the adult Korean population. J Allergy Clin Immunol. 2014; 133:1048–1055.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download