Abstract
Eosinophilic esophagitis (EoE) is a clinicopathologic disease characterised by esophageal dysfunction and eosinophil-predominant inflammation. Diagnosing EoE in children is particularly challenging as they tend to present with nonspecific symptoms and their mucosal specimens may have less than the threshold number of eosinophils. Reluctance to subject children to multiple endoscopic procedures and anesthesia often results in treatment with a proton pump inhibitor (PPI) first to evaluate clinical response. This approach presents a problem as a diagnosis of EoE may be missed. Here we present a case of a child with severe EoE whose initial biopsy did not show esophageal eosinophilia but progressed on to advance dis ease despite clinical response to PPI therapy.
Eosinophilic esophagitis (EoE) is defined as a chronic immune or antigen-mediated esophageal disease characterised by esophageal dysfunction and eosinophil-predominant inflammation [1]. Unfortunately, diagnosing EoE in children is often challenging. Apart from presenting with subtle symptoms that may be missed by unsuspecting parents and physicians, infants may present with reflux-like symptoms, feeding difficulties and growth failure, all of which feature in other common childhood conditions, including gastro-esophageal reflux disease (GERD) [23]. There is also increasing recognition of a subgroup of patients with features of EoE who achieve clinicopathological remission on proton pump inhibitor (PPI) therapy and are described as having PPI-responsive esophageal eosinophilia (PPI-REE).
This case describes a child with severe EoE whose initial biopsy did not show esophageal eosinophilia but progressed on to advance disease despite clinical response to PPI therapy. Informed consent was obtained from the patient.
A 3-year-old boy presented at 12 months of age with hematemesis and faltering growth. Prior to this, he was diagnosed with GERD when he was 2 months old because of persistent vomiting. His symptoms did not improve with histamine receptor 2 antagonist therapy and his weight and height were below the 3rd centile. Following an episode of coffee-ground vomitus at 12 months, he was prescribed a PPI with subsequent resolution of all symptoms and good catch-up growth.
An upper gastrointestinal endoscopy was performed at 18 months when an attempt to stop PPI therapy resulted in recurrence of vomiting and hematemesis. Endoscopy revealed furrowing at the midesophagus with mucosal nodularity and friability at the distal esophagus. Histology from multiple biopsy sites showed nonspecific esophagitis and <5 eosinophils per high-power field (HPF). Of note, histology from the distal esophagus demonstrated greater degree of inflammation compared to midesophagus which was in keeping with GERD. He was treated for reflux esophagitis and remained asymptomatic with good growth while on PPI although symptoms recurred whenever treatment was stopped. He later developed atopic dermatitis, allergic rhinitis and immediate allergic reactions to egg and hazelnut.
The patient was strictly avoiding egg and all nuts when he was seen at 3 years of age. He was consuming cow's milk formula regularly and was asymptomatic as long as he was on PPI therapy. On examination, he was alert and well-thrived with weight and height at 75th–90th percentile for age. His skin prick test was positive for egg, hazelnut, and house dust mite (egg white 7.5 mm, hazelnut 3 mm, house dust mite 4 mm, histamine 5 mm, normal saline 0 mm). Repeat endoscopy revealed multiple longitudinal furrows and concentric rings, giving a cobblestone appearance to the mid and distal esophagus (Fig. 1). Biopsy from the midesophagus demonstrated up to 40 eosinophils/HPF including eosinophilic microabscesses (Fig. 2), while the distal esophagus showed up to 20 eosinophils/HPF, confirming the diagnosis of EoE. The patient was commenced on an elimination diet (avoiding cow's milk, soy, egg, wheat, and nuts) and referred to a dietician while continuing PPI. Over the next 4 weeks, he was noted to show greater willingness to try new foods, required less time to finish meals and drank less during meal times. He remains well on an elimination diet.
While symptoms of esophageal dysfunction in the presence of ≥15 eosinophils/HPF confirms the diagnosis of EoE [1], the converse may not be true and paucity of eosinophils on esophageal biopsy does not exclude EoE in children, particularly those on PPIs. Current guidelines recommend biopsies to be performed when the patient has been treated with PPI for at least 6 to 8 weeks to rule out esophageal eosinophilia caused by GERD and PPI-REE [34]. A diagnosis of EoE can be made if histology shows esophageal eosinophilia despite PPI therapy and other causes have been excluded. Reluctance to subject children to multiple endoscopic procedures supports this approach, leading to most children presenting with vomiting and regurgitation being treated with PPIs before proceeding with further investigations. Unfortunately, our case, as well as other reports [56], suggest this approach may result in a diagnosis of EoE being missed.
It has been recognised that some patients with EoE who are treated with PPIs might have less than the threshold number of eosinophils. Possible explanations include inadequate biopsy specimens, sampling error, or partial treatment response [1]. Furthermore, some patients show clinicopathological remission on PPI therapy and are described as having PPI-REE. These patients have clinical, endoscopic, and histologic features that are indistinguishable from EoE except that PPIs may reverse Th2-mediated inflammation [78]. However, this PPI responsiveness can be transient as noted in reports of children who suffer clinicopathological relapses despite showing initial improvement on PPI therapy [56]. Dohil et al. [5] described 4 children with severe esophageal eosinophilia who initially responded to PPI therapy but later relapsed into EoE. A possible explanation was that the anti-inflammatory effects of PPIs was insufficient to overcome antigen-driven inflammation. It appears PPI-responsiveness does not exclude EoE and performing a baseline endoscopic assessment to document presence of tissue eosinophilia prior to PPI therapy may be necessary in some patients. Furthermore, serial histological assessments to look for progression to EoE may be needed even if there is clinicopathological response to PPI therapy.
Identifying patients who would benefit from baseline and serial endoscopic assessments can be challenging. One of the difficulties often faced is distinguishing EoE from GERD. Apart from overlapping clinicopathological features, these 2 conditions frequently coexist. Typical symptoms of dysphagia and food impaction would suggest EoE but children are often quick to adapt their eating habits to cope with esophageal dysfunction and this compensatory behaviour may be missed by parents and physicians [1]. Moreover, correlation of symptom severity with degree of inflammation has not been consistently demonstrated [9101112], emphasizing the need for serial endoscopic assessments to monitor for histological progression.
This case highlights some of the challenges and controversies of diagnosing EoE. In particular, normal histologic features in children on PPI therapy do not exclude EoE while atypical features of GERD, such as recurrent hematemesis and the inability to wean PPI therapy, should prompt the clinician to consider baseline and serial endoscopic assessments.
Figures and Tables
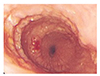 | Fig. 1Endoscopic image of the esophagus performed at 3 years of age, demonstrating concentric rings and nodularity in the mid and distal esophagus, and easy contact bleeding. |
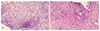 | Fig. 2(A) Biopsy taken from the mid esophagus at 18 months of age showing no pathological findings. (B) Biopsy taken from the mid esophagus at 3 years of age showing marked increase in eosinophils of up to 40 eosinophils/high-power field, including the formation of eosinophilic microabscesses. Scale bar represents 50 µm. |
References
1. Liacouras CA, Furuta GT, Hirano I, Atkins D, Attwood SE, Bonis PA, Burks AW, Chehade M, Collins MH, Dellon ES, Dohil R, Falk GW, Gonsalves N, Gupta SK, Katzka DA, Lucendo AJ, Markowitz JE, Noel RJ, Odze RD, Putnam PE, Richter JE, Romero Y, Ruchelli E, Sampson HA, Schoepfer A, Shaheen NJ, Sicherer SH, Spechler S, Spergel JM, Straumann A, Wershil BK, Rothenberg ME, Aceves SS. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol. 2011; 128:3–20.e6.

2. Furuta GT, Liacouras CA, Collins MH, Gupta SK, Justinich C, Putnam PE, Bonis P, Hassall E, Straumann A, Rothenberg ME. First International Gastrointestinal Eosinophil Research Symposium (FIGERS) Subcommittees. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology. 2007; 133:1342–1363.

3. Muraro A, Werfel T, Hoffmann-Sommergruber K, Roberts G, Beyer K, Bindslev-Jensen C, Cardona V, Dubois A, duToit G, Eigenmann P, Fernandez Rivas M, Halken S, Hickstein L, Høst A, Knol E, Lack G, Marchisotto MJ, Niggemann B, Nwaru BI, Papadopoulos NG, Poulsen LK, Santos AF, Skypala I, Schoepfer A, Van Ree R, Venter C, Worm M, Vlieg-Boerstra B, Panesar S, de Silva D, Soares-Weiser K, Sheikh A, Ballmer-Weber BK, Nilsson C, de Jong NW, Akdis CA. EAACI Food Allergy and Anaphylaxis Guidelines Group. EAACI food allergy and anaphylaxis guidelines: diagnosis and management of food allergy. Allergy. 2014; 69:1008–1025.

4. Papadopoulou A, Koletzko S, Heuschkel R, Dias JA, Allen KJ, Murch SH, Chong S, Gottrand F, Husby S, Lionetti P, Mearin ML, Ruemmele FM, Schäppi MG, Staiano A, Wilschanski M, Vandenplas Y. ESPGHAN Eosinophilic Esophagitis Working Group and the Gastroenterology Committee. Management guidelines of eosinophilic esophagitis in childhood. J Pediatr Gastroenterol Nutr. 2014; 58:107–118.

5. Dohil R, Newbury RO, Aceves S. Transient PPI responsive esophageal eosinophilia may be a clinical sub-phenotype of pediatric eosinophilic esophagitis. Dig Dis Sci. 2012; 57:1413–1419.

6. Schroeder S, Capocelli KE, Masterson JC, Harris R, Protheroe C, Lee JJ, Furuta GT. Effect of proton pump inhibitor on esophageal eosinophilia. J Pediatr Gastroenterol Nutr. 2013; 56:166–172.

7. Molina-Infante J, Rivas MD, Hernandez-Alonso M, Vinagre-Rodríguez G, Mateos-Rodríguez JM, Dueñas-Sadornil C, Perez-Gallardo B, Ferrando-Lamana L, Fernandez-Gonzalez N, Bañares R, Zamorano J. Proton pump inhibitor-responsive oesophageal eosinophilia correlates with downregulation of eotaxin-3 and Th2 cytokines overexpression. Aliment Pharmacol Ther. 2014; 40:955–965.

8. Kedika RR, Souza RF, Spechler SJ. Potential anti-inflammatory effects of proton pump inhibitors: a review and discussion of the clinical implications. Dig Dis Sci. 2009; 54:2312–2317.

9. Bassett J, Maydonovitch C, Perry J, Sobin L, Osgard E, Wong R. Prevalence of esophageal dysmotility in a cohort of patients with esophageal biopsies consistent with eosinophilic esophagitis. Dis Esophagus. 2009; 22:543–548.

10. Mulder DJ, Hurlbut DJ, Noble AJ, Justinich CJ. Clinical features distinguish eosinophilic and reflux-induced esophagitis. J Pediatr Gastroenterol Nutr. 2013; 56:263–270.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download