Abstract
Population aging is a global issue, but is estimated to be more rapid and dramatic in Asian countries. In the past, allergy might have been a minor concern in the elderly (usually defined as ≥65 years). However, recent series of epidemiologic studies indicate that allergic diseases are more prevalent than expected in the aged population. Furthermore, they pose significant impact on quality of life and socioeconomic costs. The burden may also increase in the elderly, due to frequent comorbidities and treatment-related complications. The Korean Longitudinal Study on Health and Aging (KLoSHA) cohort study is one of major research projects on the epidemiology of common geriatric disease conditions in Korea. In this review, we summarized the baseline findings on the prevalence, risk factors, comorbidities and impact of geriatric respiratory allergic conditions in the phase I KLoSHA cohort study.
Population aging is an emerging issue in every part of the world. Recent series of reports from the National Institute on Aging, U.S. Census Bureau have already indicated unprecedented rate of aging [1]. According to the World Population Prospects 2017 Revision data (https://esa.un.org/unpd/wpp/), Eastern Asia is aging more rapidly amongst continents and is estimated to be at the top in 2050s (Fig. 1A). Particularly, the rise is expected to be more dramatic in a few Asia-Pacific countries like Korea, Singapore, or Thailand (Fig. 1B).
In the past, allergy had been a minor health concern in the elderly, unlike in children. However, recent epidemiological studies revealed that allergic diseases are considerably prevalent in the aged population [234567]. Although most allergic diseases may not directly cause mortality, they pose considerable impact on quality of life and considerable burden to the society [8910]. The burden is aggravated by comorbidities and treatment-related side effects, which are frequent in the elderly subjects [7]. Thus, considering the rapid trend of population aging, there is a clear and urgent need for further research focused on this old but new problem.
Korea is one of the most rapidly aging countries in the world. The ratio of elderly (≥65 years) per younger (15–64 years) population, termed as old-age dependency ratio, may exceed 50% within just 2 decades (Fig. 1B). However, until recently, there has been very scanty data available regarding the epidemiology of allergic conditions in Korean elderly persons. The Korean Longitudinal Study on Health and Aging (KLoSHA) cohort study is one of major research projects to examine the epidemiology of common geriatric disease conditions in Korea. It is a population-based prospective cohort study of Korean elderly subjects, based on a single large urban city, Seongnam, in a metropolitan area. A total of 1,000 elderly participants were recruited from the urban community, using random sampling methods to represent the community population. The KLoSHA was designed as a comprehensive multidisciplinary study, thus allowing interdisciplinary collaboration, and included protocols for assessing atopy, asthma, rhinitis, and cough (Table 1) [11].
The cohort study has following aims in general: (1) to estimate the prevalence, incidence and progression of common geriatric diseases, (2) to determine risk factors for common geriatric diseases and to develop possible preventive strategies, (3) to investigate the impact of common geriatric diseases on the quality of life and general health status, (4) to evaluate the levels of health and functional status of the Korean elderly and to track the changes in these characteristics over time, and (5) to establish a uniform database for subsequent studies, and to plan and evaluate interventions [11].
The baseline phase I and subsequent phase II surveys were completed in 2005–2006 and in 2011, respectively. In phase I survey, atopic sensitization and allergic respiratory conditions were assessed for the prevalence, risk factors, and relationships with comorbidities and quality of life. In this review, we summarized main findings from the phase I survey.
Asthma is a complex condition particularly in the elderly, involving multiple risk factors and mechanisms [12]. However, in terms of prevalence and burden, asthma is already major chronic disease in the elderly [1213141516]. Recent series of surveys and literature reviews indicated that prevalence of asthma is higher in the elderly than previously thought [71213141516]. Of note, asthma prevalence frequently increased with aging in Asian older adult populations (termed as “second peak” in asthma prevalence) [17]; these findings warrant longitudinal investigation to determine whether it indicates the incidence of “late-onset asthma” in adults. In cross-sectional studies, risk factors like smoking, occupation, rhinitis/rhinosinusitis, obesity, or IgE sensitization to Staphylococcus aureus enterotoxins were suggested [717].
Prevalence of atopy, which is a traditional risk factor for asthma in children, has been variably reported in studies of elderly asthma patients, but its impact on asthma activity and severity seems much less than in younger patients [718]. In the KLoSHA cohort, atopy was found only in 23.7% of patients with current asthma (defined as positive responses to both ever asthma and current wheeze in the questionnaire survey), which was not significantly different from that in nonasthmatic controls (17.4%, p = 0.11) [19].
Recent epidemiological evidence suggested considerable relationships between asthma and obesity as overall, but not specifically in the elderly [20]. Aging is frequently associated with increased abdominal fat mass and reduced appendicular skeletal muscle mass, termed as sarcopenic obesity. It leads to deterioration in physical functions but also metabolic impairment [21]. Thus, asthma-obesity relationship is supposed to be more complex in the elderly (Fig. 2). We analysed the associations between asthma and obesity and/or sarcopenia, using conventional anthropometric indices like body mass index (BMI) and waist circumference (WC) but also computed tomography (CT)-measured abdominal visceral and subcutaneous fat areas and dual energy X-ray absorptiometry-measured regional fat and fat-free mass [19]. Current asthma was significantly associated with BMI (highest vs. lowest quartile; odds ratio [OR], 4.51; 95% confidence interval [CI], 2.01–10.13; p < 0.001) and WC (high vs. low [cutoff values: 87 cm in men and 85 cm in women] [22]; OR, 1.92; 95% CI, 1.05–3.52; p = 0.035). Interestingly, current asthma had significant relationships with reduced appendicular fat-free mass in elderly subjects ≥25.0 kg/m2, suggesting possible roles of sarcopenic obesity in geriatric asthma. In a recent study of 2,000 elderly subjects in Colombia, sarcopenia was twice more prevalent in asthma-chronic obstructive pulmonary disease patients than controls (11.2% vs. 6.5%, p = 0.004) [23].
In abdomen CT, subcutaneous fat area, but not visceral fat area, was significantly related with current asthma [19]. This finding was rather unexpected, as a major pathophysiological link was supposed to be systemic inflammatory responses to adipokine secretion from visceral fat tissues [24]. Similarly to our findings [19], another study of 3,205 Korean adult subjects recruited from a health check-up center found that subcutaneous abdominal fat amount had significant associations with airway hyperresponsiveness to methacholine [25]. However, in a recent population-based study of 6,178 children in the Netherlands, subcutaneous fat mass (measured by abdominal ultrasound) was not significantly associated with any of asthma-related outcomes including airway resistance, fractional exhaled nitric oxide (FeNO), wheezing and asthma. Rather, preperitoneal fat mass had significant relationships with FeNO levels [26]. As the distribution of BMI is considerably different between Asian and Western populations (i.e., a mean BMI in Korean adult population is just around 23–24 kg/m2) [19], these discrepancies warrant further longitudinal investigation and validation in different ethnic and/or age groups to determine exact effects of abdominal obesity on asthma.
Rhinitis is often considered as a trivial problem in the elderly, but it is highly prevalent and significantly associated with impairment in quality of life [2728]. In the KLoSHA cohort population, the prevalence of current rhinitis was 25.6% and only decreased after age of 90 years. In the short-form 36 (SF-36) questionnaire, current rhinitis was independently related to a decrease in quality of life, particularly in the physical aspects [27]. Atopy was found only in 18.8% of subjects with current rhinitis, and was not significantly associated with rhinitis as overall [27]. Among rhinitis-related symptoms, however, atopy was related with nasal itch (OR, 1.84; 95% CI, 1.04–3.25; p = 0.035) and rhinorrhoea (OR, 1.53; 95% CI, 1.02–2.29; p = 0.039), but not significantly with sneeze or nasal obstruction [27]. Of note, despite its mostly nonatopic nature, rhinitis was significantly associated with asthma [27].
Similar findings were observed in a nationwide survey in Portugal (prevalence of current rhinitis: 29.8%; 95% CI, 28.4%–31.3%) [28]. Also, a strong relationship between asthma and rhinitis was found (OR, 13.86; 95% CI, 10.66–18.02), and their associations became stronger with persistence and increasing severity of rhinitis [6]. However, atopic sensitization was not assessed in the Portuguese survey [28]. Thus, the relationships between rhinitis and asthma warrant further mechanistic explanation in the elderly.
Nature of elderly rhinitis without atopy is not clear, and vasomotor rhinitis may be a predominant form [29]. However, local allergic rhinitis is another possible consideration. In a study of 219 elderly subjects with rhinitis in Poland, 21.0% were found to have local allergic rhinitis (defined by history of allergy, positive nasal examination findings, and the presence of nasal IgE in nasal lavage) [30]. In a small comparative study between younger and older patients with a suspected clinical diagnosis of perennial allergic rhinitis (n = 48), the presence of specific IgE to Dermatophagoides pteronyssinus (either by skin prick testing or serum IgE assays) showed poorer predictability for D. pteronyssinus nasal challenge tests in older adults (>60 years) than in younger counterparts (20–59 years), suggesting a considerable prevalence of local allergic rhinitis in the elderly [31].
Environmental factors may also influence the epidemiology of geriatric rhinitis. Exposure to urban environment has been frequently associated with allergies in children and younger adults [1732]. However, effects of urbanization have rarely been reported in the elderly. We compared the prevalences of rhinitis, rhinoconjunctivitis and current wheeze in the KLoSHA cohort (urban sample) with those in the elderly subjects from Changwon-Sancheong community population cohort (semiurban and rural samples) in Korea [33]. Two cohort studies were conducted during a similar period (2005–2007) and utilized the same questionnaire protocols [2733]. The degree of urbanization (urban, semiurban, and rural) was calculated from different indices in the 2005 Population and Housing Census database of Korea, including total population number, population density, total number of houses, housing density, and proportion of apartment housing [34]. Notably, despite a cross-sectional design, a significant urban-rural gradient was observed for current rhinitis and rhinoconjunctivitis (Fig. 3), whereas it was not for current wheeze [35]. In multivariate logistic regression, urban residence (vs. rural residence) was the most significant factor associated with rhinitis and rhinoconjunctivitis [35]. In the nationwide survey in Portugal, current rhinitis was slightly more prevalent in urban dwelling elders than in rural counterparts (30.7% vs. 27.6%, p = 0.082) [28]. In a survey in Poland, perennial allergic rhinitis was significantly more prevalent in the elderly subjects living in urban area (urban vs. rural: 24.3% vs. 10.0%, p < 0.01) [36]. Considering that rhinitis is a nasal neuronal reflex to environmental or inflammatory stimuli, these findings collectively suggest that urban factors contribute to elderly rhinitis, and that control of such environmental factors might also help the management of rhinitis in elderly patients.
Based on cross-sectional studies, roles of inhalant allergen sensitization (termed as atopy) in respiratory symptoms and disease conditions are less evident in the elderly than in children [37]. In the KLoSHA cohort, atopic status (assessed using skin prick testing with 12 common inhalant allergens) had a prevalence of 17.3%, but did not have significant associations with asthma, rhinitis or chronic cough [192738]. Similarly, in the Changwon-Sancheong cohort study of Korean adults, the prevalence of atopy was only 11.8% among the entire age groups and less than 10% in the elderly group [33]. However, considering high prevalence of atopy and allergies in children and young adults, the epidemiology may change within decades. Longitudinal studies will confirm the natural history and clinical relevance of atopy in the elderly.
Skin reactivity may decrease with aging [39]; thus, the use of skin prick testing is often limited due to concern of possible false-negative responses. In the KLoSHA cohort population, we examined whether skin reactivity (wheal size) to histamine could be influenced by age, but found that the age-related decrease in the skin reactivity was only apparent among females [40]. In males, histamine wheal size did not significantly differ even in the subjects older than 90 years. In multivariate logistic regression analyses, male sex was the strongest predictor for high histamine skin reactivity (defined as wheal size ≥4 mm) [40]. Meanwhile, allergen-induced wheal size did not significantly differ between different age or sex groups. The reasons for the age-related patterns and discrepancies were not clear in cross-sectional analyses, and hopefully longitudinal follow-ups could reveal host or environmental factors underlying the findings.
Cough is a symptom not highly specific to allergic conditions, but is frequently associated with respiratory allergies like asthma and rhinitis [4142]. Of note, subacute or chronic cough is more prevalent in the elderly, while acute cough is more frequent in younger adults in the community [43]. Predominance of older patients is also observed in specialist clinics [4445]. In a recent survey of 10,032 patients with chronic cough from 11 referral clinics around the world, the most common age for presentation was 60–69 years [44]. Enhanced responses to capsaicin were significantly associated with old age preponderance in women [45]. However, reasons for cough persistence and hypersensitivity in older subjects warrant further investigation. In the KLoSHA cohort, we postulated roles of comorbid conditions [38]. Among 70 disease conditions common in the elderly (defined by physician diagnosis history and/or laboratory tests), asthma, allergic rhinitis, constipation, and poorly controlled diabetes mellitus (represented as glycated hemoglobin > 8%) showed significant relationships with chronic persistent cough [38]. Considering cross-sectional design and possible confounding effects from medication (like codeine or angiotensin converting enzyme inhibitors), the observed relationships with constipation or poorly controlled diabetes mellitus need further validation and mechanistic explanation. However, interestingly, we also observed similarly significant associations between chronic cough and diabetes mellitus (13.1% in chronic cough vs. 6.1% in no current cough; p < 0.001) in the Korean National Health and Nutrition Examination Survey 2010–2012 survey [43].
Cough poses a considerable impact on quality of life. In the KLoSHA cohort database, using SF-36 questionnaire, we observed that chronic persistent cough is independently associated with deterioration in quality of life, in both of physical and mental components [38]. Mental component score was worse in chronic persistent cough than in stroke, diabetes mellitus or hypertension, highlighting the epidemiological significance of chronic cough in the elderly (Fig. 4).
We also examined the relationships between blood eosinophilia (defined by eosinophils > 450/mm3) and allergic respiratory conditions including chronic persistent cough (Won HK et al. unpublished data). Chronic persistent cough showed a significant association with blood eosinophilia (OR, 4.53; 95% CI, 1.55–13.27; p = 0.006), which was stronger than asthma or rhinitis. The association between chronic cough and blood eosinophilia was independent of atopy, suggesting pathogenic roles and possible clinical utility of blood eosinophils for chronic cough in the elderly.
In the KLoSHA phase I study, we obtained a brief snapshot of the prevalence and possible risk factors of common respiratory allergic conditions among the Korean elderly. We found that asthma, rhinitis and chronic cough are more prevalent than expected, and they were significantly related to impairment in quality of life in the elderly. Findings on complex relationships with abdominal obesity, sarcopenia, comorbidities, or urban environments suggest that multidisciplinary approach is necessary to further understand the diseases. In phase II study, we hope to get more information about incidence, remission, and identify further risk factors of geriatric allergic conditions.
Figures and Tables
 | Fig. 1Trends and prospects of old-age dependency ratio (ratio of population aged ≥65 years per 100 population aged 15–64). (A) Comparison by continents. (B) Comparison by countries. Custom data were acquired from World Population Prospects 2017, Population Division, ©(2017) United Nations ([cited 2017 Oct 16]. Available from: https://esa.un.org/unpd/wpp/DataQuery/). Reprinted with the permission of the United Nations. |
 | Fig. 3Urban-rural gradient in the prevalence of rhinitis and rhinoconjunctivitis in the elderly. Reprinted from Song et al. Ann Allergy Asthma Immunol 2015;114:455-61, with permission of Elsevier [35]. |
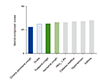 | Fig. 4Mental component scores among elderly subjects with chronic persistent cough and other conditions. HbA1c, glycated hemoglobin. Figure was drawn using the original data published in Song et al. PLoS One 2013;8:e78081 [38]. |
Table 1
Survey items included in the Korean Longitudinal Study on Health and Aging

Modified from Park et al. Psychiatr Investig 2007;4:84-95 [11].
References
1. He W, Goodkind D, Kowal P. An aging world: 2015. International population reports, P95/16-1. Washington, DC: U.S. Government Publishing Office;2016.
3. Möhrenschlager M, Ring J. Food allergy: an increasing problem for the elderly. Gerontology. 2011; 57:33–36.

4. Oraka E, Kim HJ, King ME, Callahan DB. Asthma prevalence among US elderly by age groups: age still matters. J Asthma. 2012; 49:593–599.

5. Wüthrich B, Schmid-Grendelmeier P, Schindler C, Imboden M, Bircher A, Zemp E, Probst-Hensch N. Prevalence of atopy and respiratory allergic diseases in the elderly SAPALDIA population. Int Arch Allergy Immunol. 2013; 162:143–148.

6. Pite H, Pereira AM, Morais-Almeida M, Nunes C, Bousquet J, Fonseca JA. Prevalence of asthma and its association with rhinitis in the elderly. Respir Med. 2014; 108:1117–1126.

7. Song WJ, Cho SH. Challenges in the management of asthma in the elderly. Allergy Asthma Immunol Res. 2015; 7:431–439.

8. Smith AM, Villareal M, Bernstein DI, Swikert DJ. Asthma in the elderly: risk factors and impact on physical function. Ann Allergy Asthma Immunol. 2012; 108:305–310.

9. Supporting Asthma Self-Management Behaviors Among Aging Adults (SAMBA) investigators. O'Conor R, Martynenko M, Gagnon M, Hauser D, Young E, Lurio J, Wisnivesky JP, Wolf MS, Federman AD. A qualitative investigation of the impact of asthma and self-management strategies among older adults. J Asthma. 2017; 54:39–45.
10. Kim CY, Park HW, Ko SK, Chang SI, Moon HB, Kim YY, Cho SH. The financial burden of asthma: a nationwide comprehensive survey conducted in the republic of Korea. Allergy Asthma Immunol Res. 2011; 3:34–38.

11. Park JH, Lim S, Lim JY, Kim KI, Han MK, Yoon IY, Kim JM, Chang YS, Chang CB, Chin HJ, Choi EA, Lee SB, Park YJ, Paik NJ, Kim TK, Jang HC, Kim KW. An overview of the Korean longitudinal study on health and aging. Psychiatry Investig. 2007; 4:84–95.
12. Asthma in Elderly workshop participants. Hanania NA, King MJ, Braman SS, Saltoun C, Wise RA, Enright P, Falsey AR, Mathur SK, Ramsdell JW, Rogers L, Stempel DA, Lima JJ, Fish JE, Wilson SR, Boyd C, Patel KV, Irvin CG, Yawn BP, Halm EA, Wasserman SI, Sands MF, Ershler WB, Ledford DK. Asthma in the elderly: current understanding and future research needs--a report of a National Institute on Aging (NIA) workshop. J Allergy Clin Immunol. 2011; 128:3 Suppl. S4–S24.

14. Yáñez A, Cho SH, Soriano JB, Rosenwasser LJ, Rodrigo GJ, Rabe KF, Peters S, Niimi A, Ledford DK, Katial R, Fabbri LM, Celedón JC, Canonica GW, Busse P, Boulet LP, Baena-Cagnani CE, Hamid Q, Bachert C, Pawankar R, Holgate ST. Asthma in the elderly: what we know and what we have yet to know. World Allergy Organ J. 2014; 7:8.

16. Makino S. Asthma in the elderly and aging societies in Asia Pacific. Asia Pac Allergy. 2012; 2:1–2.

17. Song WJ, Wong GW. Changing trends and challenges in the management of asthma in Asia. J Allergy Clin Immunol. 2017; 09. 27. [Epub]. DOI: 10.1016/j.jaci.2017.09.008.

18. Song WJ, Sintobin I, Sohn KH, Kang MG, Park HK, Jo EJ, Lee SE, Yang MS, Kim SH, Park HK, Kwon YE, Kim TB, Kim SH, Park HW, Chang YS, Lee BJ, Jee YK, Choi BW, Bachert C, Cho SH. Staphylococcal enterotoxin IgE sensitization in late-onset severe eosinophilic asthma in the elderly. Clin Exp Allergy. 2016; 46:411–421.

19. Song WJ, Kim SH, Lim S, Park YJ, Kim MH, Lee SM, Lee SB, Kim KW, Jang HC, Cho SH, Min KU, Chang YS. Association between obesity and asthma in the elderly population: potential roles of abdominal subcutaneous adiposity and sarcopenia. Ann Allergy Asthma Immunol. 2012; 109:243–248.

20. Beuther DA, Sutherland ER. Overweight, obesity, and incident asthma: a meta-analysis of prospective epidemiologic studies. Am J Respir Crit Care Med. 2007; 175:661–666.
21. Lim S, Kim JH, Yoon JW, Kang SM, Choi SH, Park YJ, Kim KW, Lim JY, Park KS, Jang HC. Sarcopenic obesity: prevalence and association with metabolic syndrome in the Korean Longitudinal Study on Health and Aging (KLoSHA). Diabetes Care. 2010; 33:1652–1654.

22. Lim S, Kim JH, Yoon JW, Kang SM, Choi SH, Park YJ, Kim KW, Cho NH, Shin H, Park KS, Jang HC. Optimal cut points of waist circumference (WC) and visceral fat area (VFA) predicting for metabolic syndrome (MetS) in elderly population in the Korean Longitudinal Study on Health and Aging (KLoSHA). Arch Gerontol Geriatr. 2012; 54:e29–e34.

23. Borda MG, Celis-Preciado CA, Pérez-Zepeda MU, Ríos-Zuluaga JD, Cano-Gutiérrez CA. Sarcopenia in the elderly with a history of COPD/asthma: Results of the SABE-Bogotá study. Rev Esp Geriatr Gerontol. 2016; 09. 15. [Epub]. pii:S0211-139X(16)30102-0. DOI: 10.1016/j.regg.2016.07.003.
24. Fontana L, Eagon JC, Trujillo ME, Scherer PE, Klein S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes. 2007; 56:1010–1013.

25. Kim KM, Kim SS, Kwon JW, Jung JW, Kim TW, Lee SH, Min KU, Kim YY, Cho SH. Association between subcutaneous abdominal fat and airway hyperresponsiveness. Allergy Asthma Proc. 2011; 32:68–73.

26. den Dekker HT, Ros KPI, de Jongste JC, Reiss IK, Jaddoe VW, Duijts L. Body fat mass distribution and interrupter resistance, fractional exhaled nitric oxide, and asthma at school-age. J Allergy Clin Immunol. 2017; 139:810–818.e6.

27. Song WJ, Kim MY, Jo EJ, Kim MH, Kim TH, Kim SH, Kim KW, Cho SH, Min KU, Chang YS. Rhinitis in a community elderly population: relationships with age, atopy, and asthma. Ann Allergy Asthma Immunol. 2013; 111:347–351.
28. Morais-Almeida M, Pite H, Pereira AM, Todo-Bom A, Nunes C, Bousquet J, Fonseca J. Prevalence and classification of rhinitis in the elderly: a nationwide survey in Portugal. Allergy. 2013; 68:1150–1157.

29. Pinto JM, Jeswani S. Rhinitis in the geriatric population. Allergy Asthma Clin Immunol. 2010; 6:10.

30. Bozek A, Ignasiak B, Kasperska-Zajac A, Scierski W, Grzanka A, Jarzab J. Local allergic rhinitis in elderly patients. Ann Allergy Asthma Immunol. 2015; 114:199–202.

31. King MJ, Tamulis T, Lockey RF. Prick puncture skin tests and serum specific IgE as predictors of nasal challenge response to Dermatophagoides pteronyssinus in older adults. Ann Allergy Asthma Immunol. 2008; 101:12–17.

32. Schröder PC, Li J, Wong GW, Schaub B. The rural–urban enigma of allergy: What can we learn from studies around the world? Pediatr Allergy Immunol. 2015; 26:95–102.

33. Song WJ, Chang YS, Lim MK, Yun EH, Kim SH, Kang HR, Park HW, Tomassen P, Choi MH, Min KU, Cho SH, Bachert C. Staphylococcal enterotoxin sensitization in a community-based population: a potential role in adult-onset asthma. Clin Exp Allergy. 2014; 44:553–562.

34. Statistics Korea. Population and housing census. Daejeon: Statistics Korea;2010.
35. Song WJ, Sohn KH, Kang MG, Park HK, Kim MY, Kim SH, Lim MK, Choi MH, Kim KW, Cho SH, Min KU, Chang YS. Urban-rural differences in the prevalence of allergen sensitization and self-reported rhinitis in the elderly population. Ann Allergy Asthma Immunol. 2015; 114:455–461.

36. Bozek A, Jarzab J. Epidemiology of IgE-dependent allergic diseases in elderly patients in Poland. Am J Rhinol Allergy. 2013; 27:e140–e145.

37. Scichilone N, Augugliaro G, Togias A, Bellia V. Should atopy be assessed in elderly patients with respiratory symptoms suggestive of asthma? Expert Rev Respir Med. 2010; 4:585–591.

38. Song WJ, Morice AH, Kim MH, Lee SE, Jo EJ, Lee SM, Han JW, Kim TH, Kim SH, Jang HC, Kim KW, Cho SH, Min KU, Chang YS. Cough in the elderly population: relationships with multiple comorbidity. PLoS One. 2013; 8:e78081.

39. Skassa-Brociek W, Manderscheid JC, Michel FB, Bousquet J. Skin test reactivity to histamine from infancy to old age. J Allergy Clin Immunol. 1987; 80:711–716.

40. Song WJ, Lee SM, Kim MH, Kim SH, Kim KW, Cho SH, Min KU, Chang YS. Histamine and allergen skin reactivity in the elderly population: results from the Korean Longitudinal Study on Health and Aging. Ann Allergy Asthma Immunol. 2011; 107:344–352.

41. ERS Task Force. Morice AH, Fontana GA, Sovijarvi AR, Pistolesi M, Chung KF, Widdicombe J, O'Connell F, Geppetti P, Gronke L, De Jongste J, Belvisi M, Dicpinigaitis P, Fischer A, McGarvey L, Fokkens WJ, Kastelik J. The diagnosis and management of chronic cough. Eur Respir J. 2004; 24:481–492.
42. Plevkova J, Song WJ. Chronic cough in subjects with upper airway diseases-analysis of mechanisms and clinical applications. Asia Pac Allergy. 2013; 3:127–135.
43. Kang MG, Song WJ, Kim HJ, Won HK, Sohn KH, Kang SY, Jo EJ, Kim MH, Kim SH, Kim SH, Park HW, Chang YS, Lee BJ, Morice AH, Cho SH. Point prevalence and epidemiological characteristics of chronic cough in the general adult population: The Korean National Health and Nutrition Examination Survey 2010-2012. Medicine (Baltimore). 2017; 96:e6486.
44. Chronic Cough Registry. Morice AH, Jakes AD, Faruqi S, Birring SS, McGarvey L, Canning B, Smith JA, Parker SM, Chung KF, Lai K, Pavord ID, van den, Song WJ, Millqvist E, Farrell MJ, Mazzone SB, Dicpinigaitis P. A worldwide survey of chronic cough: a manifestation of enhanced somatosensory response. Eur Respir J. 2014; 44:1149–1155.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download