Abstract
Intralesional triamcinolone acetonide injection is indicated for multiple skin conditions such as keloid scars, alopecia areata, and hypertrophic lichen planus. Immediate hypersensitivity reaction remains uncommon. We report on a 24-year-old woman who had received multiple intralesional injections with triamcinolone acetonide (Kenacort) plus lidocaine for keloid scar treatment without any reaction for the previous 10 years. The immediate reaction occurred 15 minutes after injection, with numbness on her face and 5 minutes later with urticaria on her chest wall and upper extremities, together with hypotension (blood pressure of 90/60 mmHg). Allergology workup revealed positive skin prick test for triamcinolone acetonide (Kenacort). Skin tests for other corticosteroids (hydrocortisone, methylprednisolone, and dexamethasone), excipients (carboxymethylcellulose, benzyl alcohol, and polysorbate 80) and lidocaine were negative, including subcutaneous challenge for lidocaine and oral challenge for carboxymethylcellulose. IgE-mediated hypersensitivity reaction must be considered in cases of multiple applications of triamcinolone acetonide injection.
Steroids are widely used as anti-inflammatory drugs for different diseases, while immediate allergic reactions are uncommon. In this article, we report a case of IgE-mediated hypersensitivity following intralesional triamcinolone acetonide injection.
A 24-year-old woman with keloid scars on her upper back had been treated with multiple intralesional injections of triamcinolone acetonide plus 1% lidocaine (xylocaine) during the previous 10 years without any reaction. After her most recent treatment, she developed generalized urticaria, lip angioedema, numbness on her face, abdominal pain, vomiting, chest tightness, and hypotension 2 minutes after injection. Lidocaine hypersensitivity was suspected. She decided to stop the treatment, without any medication being prescribed for her condition. She was healthy, with no history of any other medication use and no history of drug/food allergy or atopic diseases. After 1 year she returned for intralesional injection of triamcinolone acetonide only (without lidocaine), and was scheduled once a month for this regimen. The first injection was tolerated well. The reaction occurred at the time of the second injection, with numbness on her face developing 15 minutes after injection and 5 minutes later with urticaria on her chest wall and both arms, together with hypotension (blood pressure of 90/60 mmHg). At the emergency room, her vital signs revealed blood pressure 90/60 mmHg, pulse rate 72 beats/min, respiratory rate 20 breaths/min, temperature 37.2℃, and oxygen saturation 95% on room air. Immediate treatment with an intramuscular injection of adrenaline, intravenous dexamethasone and chlorpheniramine was given, and the patient quickly recovered. Laboratory investigation showed white blood cells 13.7×109/L (neutrophils 86%, lymphocytes 11%, monocytes 2%, eosinophils 1%) and serum tryptase 5.89 ng/mL (1.9–13.5 ng/mL).
Anaphylaxis was diagnosed. The allergology workup was performed 4 weeks after the anaphylaxis event to clarify the identity of the culprit drug. Skin tests with triamcinolone acetonide, lidocaine, and the excipients carboxymethylcellulose (CMC), benzyl alcohol and polysorbate 80, were performed, together with alternative corticosteroids (hydrocortisone, methylprednisolone, and dexamethasone).
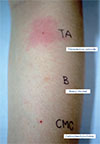
The skin test concentrations were as previously reported [12] and are summarized in Table 1. Histamine and normal saline were used as positive and negative controls, respectively. The skin prick test (SPT) was considered positive when a wheal of more than 3 mm in diameter presented after 15 minutes. If the SPT was negative, an intradermal test (IDT) was performed by injecting 0.02–0.05 mL of the reagent solution intradermally. Results were interpreted after 20 minutes, and a positive result was declared when the wheal and erythema extended more than 2 mm from the initial injection papule [2]. When SPT and IDT were negative, a provocation test was performed.
The patient had a positive SPT for triamcinolone acetonide (Table 1), which confirmed IgE-mediated hypersensitivity (Fig. 1). The SPT for the other corticosteroids, including hydrocortisone, methylprednisolone, and dexamethasone, were negative. Subcutaneous provocation with lidocaine (cumulative dose of 3.2 mL of 1% lidocaine) and oral CMC (cumulative dose of 250 mg) was well-tolerated and considered negative.
Corticosteroids are widely used in different diseases due to their anti-inflammatory and immunoregulatory effects. However, hypersensitivity reactions can occur. Corticosteroid hypersensitivity reactions can be classified into 2 categories: (1) type IV, delayed-type (T cell-mediated) [3]; and (2) type I, immediate-type (IgE-mediated). Immediate hypersensitivity reactions are relatively rare, with a prevalence of approximately 0.1% [45]. The clinical presentations vary from urticaria and angioedema to anaphylaxis [1].
Corticosteroids are classified into A, B, C, and D groups (with the latter further subdivided into D1 and D2 subgroups) based on their structural and clinical characteristics [67]. Cross-reactivity within the same group and between groups of corticosteroids has been reported, especially groups A, B, and D, according to their stereospecificity [8910]. However, some studies were unable to demonstrate cross-reactions between corticosteroids [111]. In 2011, Baeck et al. [1213] proposed a new classification based on cross-reactions and molecular structure, which divided corticosteroids into 3 groups (Table 2). Allergic reactions were found to occur more frequently in group I corticosteroids, whereas group III had the fewest cross-reactions [14].
Several case reports on triamcinolone acetonide hypersensitivity have been published, in which the culprit was either the corticosteroid [115] or its excipients [1617], including CMC, benzyl alcohol, and polysorbate 80.
In this case, triamcinolone acetonide and lidocaine were taken into account for the anaphylactic reaction at the first episode. We extensively investigated both drugs, excipients, and other corticosteroids to evaluate the cross-reactivity between groups.
Skin test results for triamcinolone acetonide were positive, whereas other corticosteroids, lidocaine, and the excipients of triamcinolone acetonide (CMC, benzyl alcohol, and polysorbate 80) were negative. The provocation test to lidocaine was negative. Instead of a subcutaneous CMC provocation test, the oral route was chosen due to the lack of a standard protocol and the irritation property of CMC.
Baker et al. [18] reported on a case series of 23 patients with suspected corticosteroid hypersensitivity. Almost all of the cases with a positive skin test had a history of anaphylaxis; intra-articular triamcinolone acetonide was the causative agent in 5 out of 8 patients. To our knowledge, the present report is the first case in Thailand and one of the few cases of confirmed IgE-mediated triamcinolone acetonide hypersensitivity via the intralesional route. Despite widespread use, reports of type I hypersensitivity to corticosteroids are rare. It is important to be aware of the risk of anaphylaxis with multiple intralesional injections of corticosteroids. We suggest that extensive allergology testing both for corticosteroids and their excipients would be valuable.
A limitation of this report is that we could not perform in vitro specific IgE for triamcinolone acetonide and CMC; however, evidence of IgE-mediated hypersensitivity from the skin test was sufficient for a definitive diagnosis.
Figures and Tables
References
1. Venturini M, Lobera T, del Pozo MD, González I, Blasco A. Immediate hypersensitivity to corticosteroids. J Investig Allergol Clin Immunol. 2006; 16:51–56.
2. Kränke B, Aberer W. Skin testing for IgE-mediated drug allergy. Immunol Allergy Clin North Am. 2009; 29:503–516.

3. Jacob SE, Steele T. Corticosteroid classes: a quick reference guide including patch test substances and cross-reactivity. J Am Acad Dermatol. 2006; 54:723–727.

4. Klein-Gitelman MS, Pachman LM. Intravenous corticosteroids: adverse reactions are more variable than expected in children. J Rheumatol. 1998; 25:1995–2002.
5. Nakamura H, Matsuse H, Obase Y, Mitsuta K, Tomari S, Saeki S, Kawano T, Kondo Y, Machida I, Shimoda T, Asai S, Kohno S. Clinical evaluation of anaphylactic reactions to intravenous corticosteroids in adult asthmatics. Respiration. 2002; 69:309–313.

6. Coopman S, Degreef H, Dooms-Goossens A. Identification of cross-reaction patterns in allergic contact dermatitis from topical corticosteroids. Br J Dermatol. 1989; 121:27–34.

8. Fulcher DA, Katelaris CH. Anaphylactoid reaction to intravenous hydrocortisone sodium succinate: a case report and literature review. Med J Aust. 1991; 154:210–214.

9. Lepoittevin JP, Drieghe J, Dooms-Goossens A. Studies in patients with corticosteroid contact allergy. Understanding cross-reactivity among different steroids. Arch Dermatol. 1995; 131:31–37.

10. Isaksson M, Bruze M, Lepoittevin JP, Goossens A. Patch testing with serial dilutions of budesonide, its R and S diastereomers, and potentially cross-reacting substances. Am J Contact Dermat. 2001; 12:170–176.

11. Rao KV, Andersen RC, O'Brien TJ. Successful renal transplantation in a patient with anaphylactic reaction to Solu-Medrol (methylprednisolone sodium succinate). Am J Med. 1982; 72:161–163.

12. Baeck M, Chemelle JA, Goossens A, Nicolas JF, Terreux R. Corticosteroid cross-reactivity: clinical and molecular modelling tools. Allergy. 2011; 66:1367–1374.

13. Baeck M, Chemelle JA, Rasse C, Terreux R, Goossens A. C(16)-methyl corticosteroids are far less allergenic than the non-methylated molecules. Contact Dermatitis. 2011; 64:305–312.

14. Baeck M, Goossens A. Immediate and delayed allergic hypersensitivity to corticosteroids: practical guidelines. Contact Dermatitis. 2012; 66:38–45.

15. Karsh J, Yang WH. An anaphylactic reaction to intra-articular triamcinolone: a case report and review of the literature. Ann Allergy Asthma Immunol. 2003; 90:254–258.

16. Montoro J, Valero A, Elices A, Rubira N, Serra-Baldrich E, Amat P, Malet A. Anaphylactic shock after intra-articular injection of carboxymethylcellulose. Allergol Immunopathol (Madr). 2000; 28:332–333.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download