Abstract
Second-generation antihistamines are widely prescribed for the control of symptoms of allergic inflammation such as itchy hives, coryza, and itchy eyes. In rare circumstances, these drugs might provoke allergic inflammation. Hypersensitivity to bepotastine besilate, a second-generation antihistamine has never been reported. A 17-year-old schoolgirl, whose paroxysmal itchy hives had been controlled with bepotastine, experienced aggravation of the hives. An oral provocation test confirmed her hypersensitivity to bepotastine and cross-reactivity to levocetirizine. She showed no reaction to chlorpheniramine, ketotifen, or olopatadine among the 13 antihistamines tested. While searching for an antihistamine to control her itchy hives, we found that she also exhibited cross-reactivity to various antihistamines with different chemical structures from that of bepotastine, which is not predicted according to the chemical classification of antihistamines. We report a case of hypersensitivity to bepotastine besilate in a patient with chronic spontaneous urticaria.
Bepotastine besilate is a widely prescribed, second-generation antihistamine for the control of symptoms of allergic inflammation such as itchy hives, coryza, and itchy eyes. It suppresses allergic inflammation through its selective H1-receptor antagonist activity [1].
Adverse drug reactions (ADRs) to antihistamines are frequently encountered in clinical practice. ADRs are often associated with the pharmacological properties of these drugs and are less likely to be caused by second-generation antihistamines [2]. ADRs to antihistamines not related to the pharmacological properties are rarely found in the literature. Although prescribed mainly for the control of allergic inflammation, in rare circumstances, these drugs can provoke allergic inflammation resulting in itchy hives and even anaphylaxis [3456].
We report a case of a schoolgirl with an immediate-type hypersensitivity reaction to bepotastine besilate and cross-reactivity to other antihistamines.
A 17-year-old girl visited our clinic for itchy hives that had been aggravated by medication. Two years before, she had had itchy hives from time to time, which disturbed her school life. Using a single dose of medication as needed (Bepotastine besilate 10 mg, Talion, Dong-A ST, Seoul, Korea), her hives were controlled for hours or days. One month before her visit to our clinic, she felt that the medication was not working properly, and noted that her hives were aggravated and lasted longer after taking the usual medication.
She denied other disease conditions. She avoided using nonsteroidal anti-inflammatory drugs, which had aggravated her hives on previous occasions.
The patient was given an oral provocation test when she had no hives. Itchy hives appeared 20 min after administration of 10-mg bepotastine besilate, and the hives disappeared spontaneously in hours (Fig. 1). Various antihistamines of a different chemical class were given orally [2]. She showed a similar reaction to levocetirizine (Xyzal, UCB, Seoul, Korea). Chlorpheniramine (Peniramin, Yuhan, Seoul, Korea) provoked no skin reaction, but she complained of sleepiness that disturbed her studies.
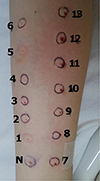
To find an effective antihistamine to control the hives without inducing an ADR, skin tests were planned with antihistamines available as a liquid [7]. In prick tests with 13 different antihistamines, only histamine as a positive control produced a positive wheal size of 30 mm × 20 mm. Intradermal tests produced negative results only to chlorpheniramine, ketotifen, and olopatadine (Table 1, Fig. 2). Ketotifen 1 mg (Ketotifen Sama, Sama Pharm, Wonju, Korea) was administered orally, and no ADR was observed.
H1 antihistamines are functionally classified into first- and second-generation antihistamines according to their lipophilicity. Most ADRs such as cognitive impairment, dry mouth, urinary retention, and weight gain are likely to be caused by first-generation antihistamines with greater lipophilicity. Others have classified H1 antihistamines historically by their chemical structures into alkylamines, piperazines, piperidines, ethanolamines, ethylenediamines, and phenothiazines [2]. Piperidines comprise most of the frequently prescribed antihistamines such as bepotastine, ketotifen, loratadine, desloratadine, ebastine, and fexofenadine.
There are several reports of hypersensitivity caused by antihistamines. Hypersensitivity to a single antihistamine resulting in hives and even anaphylaxis has been reported for cetirizine, levocetirizine, hydroxyzine, ebastine, and fexofenadine [3489]. Hypersensitivity between different classes of antihistamines has rarely been reported. Cross-reactivity between piperidines (fexofenadine, bepotastine, loratadine, ebastine) and piperazines (hydroxyzine, cetirizine) [35], and among all kinds of antihistamines [7] were reported. Fixed drug eruption by piperazine derivatives was reported [10].
In this patient, investigation of her hypersensitivity to bepotastine besilate revealed cross-reactivity with other antihistamines to which she had not been exposed. She experienced hives with orally administered levocetirizine, which is a piperazine. She had a positive intradermal test with loratadine, which is a piperidine, as is bepotastine. No skin reaction was noted to the alkylamine chlorpheniramine or to the piperidines ketotifen or olopatadine. In the previous cases of hypersensitivity to more than one antihistamine, the hypersensitivity involved structurally unrelated antihistamines [2357]. Cross-reactivity in association with hypersensitivity may be explainable and predictable according to the chemical structure for some drugs, but this may not be the case for antihistamines [11].
An oral provocation test using a therapeutic dose is confirmative in the diagnosis of an ADR. Despite the falsene-gativity [356], a negative intradermal test might be helpful for predicting negative results in an oral provocation test [78]. The concentrations of the antihistamine test reagents have not been defined yet. Here, we used antihistamines that were available in topical or intravenous formulae. They were ready-made for testing, but the concentrations of the reagents used were not comparable. Intradermal tests using various antihistamines might be useful for selecting antihistamines that do not induce hypersensitivity.
In conclusion, we report on a case of hypersensitivity to bepotastine besilate in a patient with chronic urticaria. While searching for an antihistamine to control her itchy hives, cross-reactivity to various antihistamines with different chemical structures was noted.
Figures and Tables
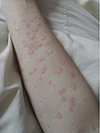 | Fig. 1The patient's right forearm. Itchy hives over the entire body were noted after an oral provocation with bepotastine besilate. |
ACKNOWLEDGEMENTS
This research was supported by a grant from Ministry of Food and Drug Safety to the Regional Pharmacovigilance Center in Jeju National University Hospital in 2016.
References
1. Williams JI, Gow JA, Klier SM, McCue SL, Salapatek AM, McNamara TR. Non-clinical pharmacology, pharmacokinetics, and safety findings for the antihistamine bepotastine besilate. Curr Med Res Opin. 2010; 26:2329–2338.

2. Simons FE, Akdis CA. Histamine and H1 antihistamines. In : Adkinson NF, Bochner BS, Burks AW, Busse WW, Holgate ST, Lemanske RF, editors. Middleton's allergy: principles and practice. Philadelphia (PA): Elsevier Saunders;2013. p. 1503–1533.
3. Rodríguez del Río P, González-Gutiérrez ML, Sánchez-López J, Nuñez-Acevedo B, Bartolomé Alvarez JM, Martínez-Cócera C. Urticaria caused by antihistamines: report of 5 cases. J Investig Allergol Clin Immunol. 2009; 19:317–320.
4. Karamfilov T, Wilmer A, Hipler UC, Wollina U. Cetirizine-induced urticarial reaction. Br J Dermatol. 1999; 140:979–980.

5. Inomata N, Tatewaki S, Ikezawa Z. Multiple H1-antihistamine-induced urticaria. J Dermatol. 2009; 36:224–227.

7. Kim YU, Lee J. Drug hypersensitivity to various antihistamines with cross-reactions. Allergy Asthma Respir Dis. 2014; 2:134–137.

8. Lee SW, Byun JY, Choi YW, Myung KB, Choi HY. Fexofenadine-induced urticaria. Ann Dermatol. 2011; 23:Suppl 3. S329–S332.

9. Choi GS, Sung JM, Lee JW, Park HJ, Ye YM, Nahm DH, Park HS. Two cases of urticaria induced by ebastine. Korean J Asthma Allergy Clin Immunol. 2009; 29:64–68.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download