Abstract
Background
Imidacloprid has been commonly used as a pesticide for crop protection and acts as nicotinic acetylcholine receptor agonists. Little information about the relationship between imidacloprid and allergy is available.
Objective
This study aims to examine the effects of imidacoprid on IgE-mediated mast cell activation.
Methods
The rat basophilic leukemia cell line RBL-2H3 (RBL-2H3 cells) were treated with 10-3 – 10-12 mol/L imidacloprid, followed by measuring the mediator production, influx of Ca2+ in IgE-activated RBL-2H3 cells, and the possible effects of imidacoprid on anti-dinitrophenyl IgE-induced passive cutaneous anaphylaxis (PCA).
Allergy occurs in individuals who have formed allergen-specific IgE antibodies following exposure and sensitization to specific allergens. Mast cells are key effector cells in IgE-mediated allergic diseases [1234]. They express the high-affinity IgE receptor FcεRI, and the binding of an allergen to IgE-FcεRI induces the increased mobilization of calcium (Ca2+), followed by the degranulation of mast cell. The activated mast cells release three classes of proinflammatory mediators: preformed granule-associated chemical mediators, such as histamine and β-hexosaminidase (β-hex); newly synthesized arachidonic acid metabolites, such as leukotrienes (LTs); and proinflammatory cytokines including tumor necrosis factor (TNF)-α and Th2 cytokines, such as interleukin (IL)-4, IL-6, and IL-13 [1567]. These mediators are critical in the development of the allergic response.
The prevalence of allergic disease have increased dramatically in the last few decades [891011]. Multiple environmental chemicals such as pesticides, solvents, and air pollutants have been associated with increased incidences of allergy [12]. Particularly, many pesticides (e.g., malathion, parathion, and methoxychlor) have been shown to enhance type 2 helper T cell (Th2) dominance and thereby play a role in the increasing trend of allergic diseases [12], nevertheless there could be another category of environmental factors, such as childhood infections, have an overwhelming and consistent negative association with allergic diseases [1314].
Imidacloprid, 1-[(6-chloro-3-pyridinyl)methyl]-N-nitro-2-imidazolidinimine, has been used as an pesticide for crop protection worldwide over the last decade [151617]. It is the best known neonicotinoid and a remarkably potent neurotoxic insecticide, which acts as nicotinic acetylcholine receptor (nAChRs) agonists [18]. Besides it is developmental toxicity, genotoxicity and chronic toxicity, there was also an attempt to explore the immunotoxicity of imidacloprid [1619202122]. Recent advances showed that imidacloprid can suppress adaptive and inflammatory immune responses [2324]. However, little information about the relationship between imidacloprid and allergy is available. In this study, the IgE-activated rat mast cell/basophil cell line RBL-2H3 (RBL-2H3 cells) was treated with imidacloprid, followed by testing the proinflammatory mediators released from the RBL-2H3 cells, Ca2+ mobilization in IgE-activated RBL-2H3 cells and vascular extravasation in IgE-induced passive cutaneous anaphylaxis (PCA) to define the possible effects of imidacloprid on IgE-mediated allergy.
The rat basophilic leukemia cell line RBL-2H3 was provided by the Type Cell Culture Collection of the Chinese Academy of Science (Shanghai, China). The imidacloprid, ketotifen fumarate salt, monoclonal anti-dinitrophenyl IgE antibody (anti-DNP IgE) were obtained from Sigma-Aldrich Co. (St. Louis, MO, USA).
Dinitrophenyl-human serum albumin (DNP-HSA) was from Biosearch Technologies Inc. (Novato, CA, USA)and the cell counting kit-8 (CCK-8) was provided by Dojindo Laboratories, (Tokyo, Japan). The vendors for the other reagents are included with the relevant assays.
The cells were cultured at 1–2×106 cells in minimum essential medium (Hyclone Laboratories, a subsidiary of GE Healthcare Life Sciences, Logan, UT, USA) supplemented with 100-U/mL penicillin, 100-µg/mL streptomycin, and 10% heat-inactivated fetal bovine serum (Gibco, a subsidary of Thermo Fisher Scientific, Waltham, MA, USA) at 37℃ under humidified atmosphere containing 5% CO2. Cells were passaged every 1–2 days.
CCK-8 assay was performed as previously described [25]. Briefly, RBL-2H3 cells were cultured in a 96-well plate and treated with varying concentrations of imidacloprid for 24 hours. Then, the supernatant of each well was removed, followed by introducing CCK-8 solution for 4 hours incubation, and the absorbance intensity was measured at 450 nm in a microplate reader (Thermo Fisher Scientific).
The RBL-2H3 cells were cultured for 24 hours to allow membrane receptors to be resynthesized, followed by replenishing with fresh 10% fetal calf serum (Gibico) complete medium containing 500-ng/mL anti-DNP IgE overnight at 37℃. After incubation overnight, cells were treated with 10-3–10-12 mol/L imidacloprid in dimethylsulfoxide (DMSO) for 8 hours, followed by stimulation with 50-µg/mL DNP-HAS for different duration of time in various assays. Cells and supernatants were collected for subjecting to various assays. Regarding to the positive control, 10-5 mol/L ketotifen fumarate salt in DMSO was used to replace imidacloprid, while the negative control group was only cultured with DMSO.
The amount of histamine released was measured by enzyme immunoassay according to the manufacturer's instructions (Cayman Chemical, Ann Arbor, MI, USA). The β-hex activity was assayed as described previously [26]. Briefly, RBL cells were stimulated with DNP-HAS in Tyrode's buffer for 30 minutes at 37℃. The supernatants were collected, and the unreleased β-hex was quantified by adding 0.5% Triton X-100 solution to the cells. Samples (50 µL) and the substrate solution (50-µL p-nitrophenyl-Nacetyl-β-D-glucosamide) was added to 96-well microtiter plate, respectively. Then, the plates were incubated at 37℃ for 60 minutes, followed by adding 200-µL stop solution (0.2-mol/L glycine, pH 10) to each well, and absorbance was recorded at 405 nm. The amount of β-hex or histamine release into media was expressed as the percentage of the total amount of β-hex or histamine originally in the cells [% release=100×(experimental β-hex or histamine release-spontaneous β-hex or histamine release)÷total cellular β-hex or histamine].
Leukotriene C4 (LTC4) was detected as described previously [27]. The supernatants were collected after various treatments of red blood cells, and the content of LTC4 was tested by using an enzyme immunoassay kit from Cayman Chemical according to the manufacturer's protocol. The amount of LTC4 into media was expressed as the release of β-hex or histamine.
After various treatments of RBL cells, the cells were activated with 50-µg/mL DNP-HAS for 8 hours, followed by collecting supernatants to test the IL-6 and TNF-α using an enzyme immunoassay kit from Abcam Inc. (Cambridge, MA, USA) according to the manufacturer's protocol, respectively.
The fluorescence assay was performed as previously described [28]. After various treatments, the RBL-2H3 cells were incubated cells with 5-µM Fluo-3/AM containing 0.05% Pluronic F127 in modified Tyrode's buffer (without CaCl2) for 30 minutes at 37℃. After washing, cells were stimulated with 50-µg/mL DNP-HAS. Fluorescence image were observed by an inverted fluorescent microscope (Nikon Eclipse Ti-U, Nikon Instruments, Kanagawa, Japan) [29]. To further evaluate the effect of imidacloprid on the intracellular calcium level, the fluorescence intensity was measured at a 488-nm excitation wavelength and a 526-nm emission wavelength with a Varioskan Flash microplate reader (Thermo Fisher Scientific).
An IgE-dependent cutaneous reaction was carried out as described previously [30]. Anti-DNP IgE (500 ng/ear) was intradermally injected into a Balb/c mouse ear. In 24 hours later, one ear of the mice were treated different concentration of imidacloprid or ketotifen fumarate salt in 100-µL DMSO for 8 hours, followed by challenging with an intravenous injection of 50-µg/mL DNP-HAS in 200-µL phosphate buffered saline containing 4% Evans blue. One hour after the antigen challenge, the mice were killed and the ears were removed to measure the amount of dye extravagated by the antigen. After overnight extraction of dye with 1 mL of 1-mmol/L potassium hydroxide and 9 mL of mixture of acetone and phosphoric acid (5:13), the intensity of absorbance was measured at 620 nm in a Varioskan Flash microplate reader (Thermo Fisher Scientific).
The effects of imidacloprid on cell viability were checked by CCK-8 assay to ensure that the decreased level of mast cell granules was not due to the cell death. Imidacloprid of 10-3–10-9 mol/L did not significantly affect the cell viability (Fig. 1). Accordingly, at least 10-3–10-9 mol/L imidacloprid was used for the subsequent studies.
It was investigated to determine whether imidacloprid treatment could inhibit mast cell degranulation by measuring the release of β-hex and histamine from RBL-2H3 cells [31]. Fig. 2A shows that 10-3–10-11 mol/L imidacloprid significantly inhibited the release of the histamine from IgE-activated RBL-2H3 at 30 minutes after DNP-HAS stimulation as ketotifen fumarate salt did. The inhibited degranulation was also confirmed by the reduced release of β-hex from the imidacloprid-treated RBL-2H3 (Fig. 2B). Thus, imidacloprid is a relatively inhibitor of mast cell β-hex and histamine production.
FcεRI activation also induces de novo synthesis of LTs, such as LTC4 which recruits neutrophils and initiates the late-phase allergic response [32]. Therefore, these newly synthesized mediators contribute to inflammation in allergic disease.
Accordingly, the effects of imidacloprid on the production of LTC4 in activated RBL-2H3 were examined. After 8 hours postactivation, enzyme immunoassays indicated that pretreatment of RBL cells with 10-3–10-11 M imidacloprid significantly inhibited the IgE-mediated production of LTC4 (Fig. 3). It was suggested that, imidacloprid also inhibited the production of LTC4 except β-hex and histamine.
Since the allergic response is induced and maintained by inflammatory substances released from mast cells [33], the effects of imidacloprid on the expression of proinflammatory cytokines IL-6 and TNF-α in the activated RBL-2H3 was examined. The enzyme immunoassays indicated that treatment of RBL cells with 10-3–10-10 mol/L imidacloprid significantly inhibited the IgE-mediated production of IL-6 (Fig. 4A) and TNF-α (Fig. 4B) after 8-hour postactivation. It suggested that, in addition to β-hex and histamine, imidacloprid can also inhibit the production of the proinflammatory IL-6 and TNF-α cytokine from the IgE-mediated atctivation of mast cells.
IgE-mediated degranulation is preceded by increased Ca2+ influx which is critical for mast cell degranulation. The possible modulating effects of imidacloprid on IgE-induced Ca2+ mobilization in mast cells was explored in this work. As shown in Fig. 5A, imidacloprid and ketotifen treatment significantly decreased the amplitude of the Ca2+ increase (indexed by the maximum change of ΔF–F0). Fluorescence image also revealed a decreased intracellular calcium level in imidacloprid-treated group (Fig. 5B). These results indicated that imidacloprid can suppress the IgE-mediated activation of RBL-2H3 cells through decreasing the intracellular Ca2+ mobilization.
Anti-DNP IgE-mediated PCA in a mouse model was used to confirm the inhibitory effect of imidacloprid in vivo. The vascular permeability was defined by the absorbance value of Evans blue dye. The control group was treated with DMSO without imidacloprid or ketotifen fumarate salt, while the naive group was not activated by IgE, prior to be treated with DMSO only. It was shown in Fig. 6A that only 10-3–10-5 mol/L imidacloprid treatment groups (with an absorbance value of 0.903 ± 0.01, 1.011 ± 0.025, 1.17 ± 0.01, respectively) have markedly lower absorbance values than the control group with an value of 1.32 ± 0.06, and as indicated in Fig. 6B, 10-4 mol/L imidacloprid significantly inhibited Evans blue dye extravasation (the pictures for the other group are not shown). Accordingly, all the results suggested that imidacloprid can inhibit IgE-dependent PCA.
In this study, the possible modulatory effects of imidacloprid on mast cell were investigated. It was shown that low concentrations of imidacloprid (10-3–10-10 mol/L) caused an inhibition of the degranulation of RBL-2H3 cells treated with imidacloprid.
Firstly, it was shown that imidacloprid significantly suppressed the release of preformed compounds, such as β-hex and histamine (Fig. 2), and the production of arachidonic acid metabolites, such as LTC4 (Fig. 3) and proinflammatory cytokines such as IL-6 and TNF-α (Fig. 4) from IgE-activated mast cells. Secondly, It was demonstrated that the degranulation of RBL-2H3 cells treated with imidacloprid, challenged with IgE/DNP-HSA, showed decreased intracellular calcium levels (Fig. 5). Therefore, imidacloprid in vitro can suppress IgE-induced degranulation of mast cells by decreasing intracellular Ca2+ mobilization in them. Partly consistent with in vitro results, the in vivo investigations showed that imidacloprid can furtherly reduce the PCA-induced Evans blue dye extravasation of ear in mice, which was also induced by IgE-activated mast cells (Fig. 6).
Multiple pesticides have been shown to play a role in the increasing trend of allergic diseases. Mice exposed to low levels of the organophosphate (OP) pesticide malathion and parathion, the organochlorine (OC) pesticide methoxychlor and the mixture of them showed significantly higher levels of degranulated mast cells than mice without exposure to these pesticide [3435]. Moreover, environmental estrogens, such as dieldrin, endosulfan, dichloroethene and so on can also induce mast cell degranulation and enhance IgE-mediated release of allergic mediators [36]. Particularly, epidemiological research show that people who exposed to higher levels of OP pesticides, OC pesticides or their combination had significantly higher levels of IgE and allergic diseases incidence [373839]. However the present study showed that 10-3–10-10 mol/L imidacloprid inhibited IgE-mediated activation of RBL-2H3 cells and 10-3–10-5 mol/L imidacloprid in 100-µL vehicle (1.28–0.0128 mg/kg) suppressed IgE-dependent PCA reaction in vivo. The maximum residual limits (MRL) for imidacloprid was earlier reported as 0.05 mg/kg, and the proposed temporary MRL 2 mg/kg for imidacloprid in rice would not raise any consumer health concerns and therefore was acceptable [40]. The dose of imidacloprid for suppressing PCA is smaller than the MRL of imidacloprid, and we think that it's effects on PCA can partly reflect the real impact of it on allergic diseases. Nevertheless, it seems that there is a complex relationship between insecticides and allergic diseases. Additionally, imidacloprid is usually combined with other environmental chemicals in the environment and tissues of human body. The effects of these environmental factors together will determine their impact on IgE-mediated mast cell granulation and allergic diseases.
To our knowledge, it is the first time to show that imidacloprid in nanomolar quantity can suppress the IgE-mediated activation of mast cells. Consequently, we speculate that imidacloprid and other neonicotinoid insecticides can play an role, at least partly in the considerably lower prevalence of allergic diseases in rural areas within one country, where these insecticides have been used more often and the people there might be exposed to higher levels of them. Additionally, it is well known that imidacloprid acts as nAChRs agonists and among the nicotinic receptors, α7 is the most commonly found nAChR subunit on immune cells [41]. The presence of nAChRs was suggested on murine bone marrow-derived mast cells, human skin mast cells and RBL-2H3 cells [424344]. It was shown that α7 nAChRs on mucosal mast cells plays an important role in maintaining intestinal immune homeostasis and is involved in the pathology of allergic disease [4445]. And it was reported that nicotine can block the mast cell activation via α7 nAChRs partly [44]. Since imidacoprid has the similar molecular structure to that of nicotine, consequently, it is possible that α7 nAChRs are also involved in imidacloprid-induced changes in mast cell function. Certainly, there is a need to further explore the inhibitory effects of imidacloprid on allergy and the underlying mechanisms.
Figures and Tables
 | Fig. 1Effect of imidacloprid on the cell viability of RBL-2H3. RBL-2H3 cells were treated with 10-3–10-9 mol/L imidacloprid for 24 hours, and cytotoxicity was measured by cell counting kit-8 assay. Each value represents the mean ± standard deviation of 3 experiments. *p > 0.05, compared with control (CON). |
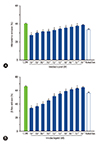 | Fig. 2Imidacloprid suppress degranulation in RBL-2H3 cells. Cells were sensitized with 500-ng/mL IgE anti-dinitrophenyl IgE overnight at 37℃, followed by pretreatment with 10-3–10-12 mol/L imidacloprid or 10-5 mol /L ketotifen fumarate salt for 8 hours before stimulation with 50-µg/mL dinitrophenyl-human serum albumin for 30 minutes. Supernatants were analyzed for enzymatic activity of histamine (A) and β-hex (B). Experiments were conducted in triplicate and expressed as mean ± standard deviation. *p <0.05, compared with control (CON). |
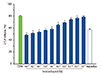 | Fig. 3Imidacloprid inhibits the production of leukotriene C4 (LTC4). The RBL-2H3 cells were sensitized with 500-ng/mL IgE anti-dinitrophenyl IgE overnight at 37℃. After washing the unbound IgE, the cells were incubated with 10-3–10-12 mol/L imidacloprid or 10-5 mol/L ketotifen fumarate salt for 8 hours. Then the cells were stimulated with 50-µg/mL dinitrophenyl-human serum albumin for another 8 hours. Supernatants were analyzed for the production of LTC4 by enzyme immunoassay following the manufacturer's instructions. Experiments were conducted in triplicate and expressed as mean ± standard error. *p < 0.05, compared with control (CON). |
 | Fig. 4Imidacloprid suppresses the expression of proinflammatory cytokines in RBL-2H3 cells. Cells were sensitized with 500-ng/mL IgE antidinitrophenyl IgE overnight at 37℃, followed by pretreatment with 10-3–10-12 mol/L imidacloprid or 10-5 mol/L ketotifen fumarate salt for 8 hours before stimulation with 50-µg/mL dinitrophenyl-human serum albumin for 8 hours. Supernatants were analyzed for the expression of interleukin (IL)-6 (A) and tumor necrosis factor (TNF)-α (B) using enzyme-linked immunosorbent assay. Experiments were conducted in triplicate and expressed as mean ± standard error. *p < 0.05, compared with control (CON). |
 | Fig. 5Imidacloprid inhibited IgE-induced Ca2+ mobilization in RBL-2H3 cells. Cells were sensitized with 500-ng/mL anti-dinitrophenyl IgE overnight at 37℃, followed by treatment with 10-3–10-12 mol/L imidacloprid or 10-5 mol/L ketotifen fumarate salt for 8 hours. The control group was cultured with dimethylsulfoxide (DMSO) without imidacloprid or 10-5 mol/L ketotifen fumarate salt, while the naïve group was not activated by IgE, prior to be treated with DMSO only. Then, the cells were incubated with Fluo 3-AM fluorescence dye for 30 minutes, followed by stimulation with 50-µg/mL dinitrophenylhuman serum albumin. Statistical analysis of the amplitude of the IgE-induced Ca2+ increase in these groups (A) was performed. The amplitude of the Ca2+ response was represented as the highest observed level of ΔF–F0. Fluorescent images of [Ca2+] were recorded using an invert microscope (B). Scale bars indicate 50 µm. Data indicate mean ± standard deviation from 3 independent experiments. *p < 0.05, compared with control (CON). |
 | Fig. 6The inhibitory effect of imidacloprid on anti-dinitrophenyl IgE-induced PCA in Balb/c mice. Anti-DNP IgE (500 ng/ear) was intradermally injected into a Balb/c mouse ear. After 24 hours later, the mice were treated with 10-4 mol/L imidacloprid or ketotifen fumarate salt in 100-µL dimethylsulfoxide for 8 hours, followed by challenging with an intravenous injection of 50-µg/mL dinitrophenyl-human serum albumin in 200-µL phosphate buffered saline containing 4% Evans blue. One hour later, the amount of Evans blue dye was measured (A) and the ear Evans blue extravasation was photographed (B). Data represent the mean ± standard deviation. *p < 0.05, compared with control (CON). |
ACKNOWLEDGEMENTS
The work was supported by the International Science & Technology Cooperation Program of China (No. 2013DFG31380, National High Technology Research and Development Program of China (863 Program; No. 2013AA102205), the Key Program of Natural Science Fundation of Jianxi Province, China (No. 20133ACB20009), and the Research Program of State Key Laboratory of Food Science and Technology (No. SKLF-ZZA-201612 and SKLF-ZZB-201302)
References
2. Galli SJ, Kalesnikoff J, Grimbaldeston MA, Piliponsky AM, Williams CM, Tsai M. Mast cells as "tunable" effector and immunoregulatory cells: recent advances. Annu Rev Immunol. 2005; 23:749–786.

3. Galli SJ, Grimbaldeston M, Tsai M. Immunomodulatory mast cells: negative, as well as positive, regulators of immunity. Nat Rev Immunol. 2008; 8:478–486.

7. Turner H, Kinet JP. Signalling through the high-affinity IgE receptor Fc epsilonRI. Nature. 1999; 402:6760 Suppl. B24–B30.
8. Burr ML, Wat D, Evans C, Dunstan FD, Doull IJ. British Thoracic Society Research Committee. Asthma prevalence in 1973, 1988 and 2003. Thorax. 2006; 61:296–299.

9. Geraldini M, Rosario NA, Riedi CA, Leopoldino BB, Rosario CS, Barkema F, Palermo F, Macedo G, KusanoL LD, Eiras NO, Robl R, Schnekenberg RP, Ribeiro TB, Macedo V. Time Trends in the prevalence of allergic diseases in childhood. J Allergy Clin Immunol. 2010; 125:2 Suppl 1. AB31.

10. Peat JK, Li J. Reversing the trend: reducing the prevalence of asthma. J Allergy Clin Immunol. 1999; 103(1 Pt 1):1–10.

11. von Mutius E. The rising trends in asthma and allergic disease. Clin Exp Allergy. 1998; 28:Suppl 5. 45–49.

12. Crinnion WJ. Do environmental toxicants contribute to allergy and asthma? Altern Med Rev. 2012; 17:6–18.
13. Maizels RM. Infections and allergy - helminths, hygiene and host immune regulation. Curr Opin Immunol. 2005; 17:656–661.

14. Yazdanbakhsh M, Wahyuni S. The role of helminth infections in protection from atopic disorders. Curr Opin Allergy Clin Immunol. 2005; 5:386–391.

15. Elbert A, Nauen R, Leicht W. Imidacloprid a novel chloronicotinyl insecticide: biological activity and agricultural importance.In : Ishaaya I, Degheele D, editors. Insecticides with novel modes of action: mechanisms and application. New York: Springer;1998. p. 50–73.
16. Gawade L, Dadarkar SS, Husain R, Gatne M. A detailed study of developmental immunotoxicity of imidacloprid in Wistar rats. Food Chem Toxicol. 2013; 51:61–70.

17. Tasei JN, Lerin J, Ripault G. Sub-lethal effects of imidacloprid on bumblebees, Bombus terrestris (Hymenoptera: Apidae), during a laboratory feeding test. Pest Manage Sci. 2000; 56:784–788.
18. Matsuda K, Shimomura M, Ihara M, Akamatsu M, Sattelle DB. Neonicotinoids show selective and diverse actions on their nicotinic receptor targets: electrophysiology, molecular biology, and receptor modeling studies. Biosci Biotechnol Biochem. 2005; 69:1442–1452.

19. Bhardwaj S, Srivastava MK, Kapoor U, Srivastava LP. A 90 days oral toxicity of imidacloprid in female rats: morphological, biochemical and histopathological evaluations. Food Chem Toxicol. 2010; 48:1185–1190.

20. Demsia G, Vlastos D, Goumenou M, Matthopoulos DP. Assessment of the genotoxicity of imidacloprid and metalaxyl in cultured human lymphocytes and rat bone-marrow. Mutat Res. 2007; 634:32–39.

21. Kapoor U, Srivastava MK, Srivastava LP. Toxicological impact of technical imidacloprid on ovarian morphology, hormones and antioxidant enzymes in female rats. Food Chem Toxicol. 2011; 49:3086–3089.

22. Mohany M, El-Feki M, Refaat I, Garraud O, Badr G. Thymoquinone ameliorates the immunological and histological changes induced by exposure to imidacloprid insecticide. J Toxicol Sci. 2012; 37:1–11.

23. Badgujar PC, Jain SK, Singh A, Punia JS, Gupta RP, Chandratre GA. Immunotoxic effects of imidacloprid following 28 days of oral exposure in BALB/c mice. Environ Toxicol Pharmacol. 2013; 35:408–418.

24. Gatne MM, Bhoir PS, Deore MD. Immunotoxicity studies of imidacloprid in rats. Toxicol Int. 2006; 13:89–92.
25. Zhang NN, Park DK, Park HJ. The inhibitory activity of atractylenolide Ш, a sesquiterpenoid, on IgE-mediated mast cell activation and passive cutaneous anaphylaxis (PCA). J Ethnopharmacol. 2013; 145:278–285.

26. Ortega E, Hazan B, Zor U, Pecht I. Mast cell stimulation by monoclonal antibodies specific for the Fc epsilon receptor yields distinct responses of arachidonic acid and leukotriene C4 secretion. Eur J Immunol. 1989; 19:2251–2256.
27. Yoshimaru T, Suzuki Y, Matsui T, Yamashita K, Ochiai T, Yamaki M, Shimizu K. Blockade of superoxide generation prevents high-affinity immunoglobulin E receptor-mediated release of allergic mediators by rat mast cell line and human basophils. Clin Exp Allergy. 2002; 32:612–618.

28. Joo HM, Nam SY, Yang KH, Kim CS, Jin YW, Kim JY. The effects of low-dose ionizing radiation in the activated rat basophilic leukemia (RBL-2H3) mast cells. J Biol Chem. 2012; 287:27789–27795.

29. Molfetta R, Gasparrini F, Peruzzi G, Vian L, Piccoli M, Frati L, Santoni A, Paolini R. Lipid raft-dependent FcepsilonRI ubiquitination regulates receptor endocytosis through the action of ubiquitin binding adaptors. PLoS One. 2009; 4:e5604.
30. Yao JH, Cui M, Li MT, Liu YN, He QH, Xiao JJ, Bai Y. Angiopoietin1 inhibits mast cell activation and protects against anaphylaxis. PLoS One. 2014; 9:e89148.

31. Han SY, Bae JY, Park SH, Kim YH, Park JH, Kang YH. Resveratrol inhibits IgE-mediated basophilic mast cell degranulation and passive cutaneous anaphylaxis in mice. J Nutr. 2013; 143:632–639.

33. Ali K, Bilancio A, Thomas M, Pearce W, Gilfillan AM, Tkaczyk C, Kuehn N, Gray A, Giddings J, Peskett E, Fox R, Bruce I, Walker C, Sawyer C, Okkenhaug K, Finan P, Vanhaesebroeck B. Essential role for the p110delta phosphoinositide 3-kinase in the allergic response. Nature. 2004; 431:1007–1011.
34. Fukuyama T, Kosaka T, Tajima Y, Ueda H, Hayashi K, Shutoh Y, Harada T. Prior exposure to organophosphorus and organochlorine pesticides increases the allergic potential of environmental chemical allergens in a local lymph node assay. Toxicol Lett. 2010; 199:347–356.

35. Nishino R, Fukuyama T, Tajima Y, Miyashita L, Watanabe Y, Ueda H, Kosaka T. Prior oral exposure to environmental immunosuppressive chemicals methoxychlor, parathion, or piperonyl butoxide aggravates allergic airway inflammation in NC/Nga mice. Toxicology. 2013; 309:1–8.

36. Narita S, Goldblum RM, Watson CS, Brooks EG, Estes DM, Curran EM, Midoro-Horiuti T. Environmental estrogens induce mast cell degranulation and enhance IgE-mediated release of allergic mediators. Environ Health Perspect. 2007; 115:48–52.

37. Duramad P, Harley K, Lipsett M, Bradman A, Eskenazi B, Holland NT, Tager IB. Early environmental exposures and intracellular Th1/Th2 cytokine profiles in 24-month-old children living in an agricultural area. Environ Health Perspect. 2006; 114:1916–1922.

38. Karmaus W, Kuehr J, Kruse H. Infections and atopic disorders in childhood and organochlorine exposure. Arch Environ Health. 2001; 56:485–492.

39. Reichrtová E, Ciznár P, Prachar V, Palkovicová L, Veningerová M. Cord serum immunoglobulin E related to the environmental contamination of human placentas with organochlorine compounds. Environ Health Perspect. 1999; 107:895–899.

40. European Food Safety Authority. Modification of the existing MRLs for imidacloprid in rice. EFSA J. 2010; 8:1589.
41. Boyd RT. The molecular biology of neuronal nicotinic acetylcholine receptors. Crit Rev Toxicol. 1997; 27:299–318.

42. Kageyama-Yahara N, Suehiro Y, Yamamoto T, Kadowaki M. IgE-induced degranulation of mucosal mast cells is negatively regulated via nicotinic acetylcholine receptors. Biochem Biophys Res Commun. 2008; 377:321–325.

43. Kindt F, Wiegand S, Niemeier V, Kupfer J, Löser C, Nilles M, Kurzen H, Kummer W, Gieler U, Haberberger RV. Reduced expression of nicotinic alpha subunits 3, 7, 9 and 10 in lesional and nonlesional atopic dermatitis skin but enhanced expression of alpha subunits 3 and 5 in mast cells. Br J Dermatol. 2008; 159:847–857.
44. Mishra NC, Rir-sima-ah J, Boyd RT, Singh SP, Gundavarapu S, Langley RJ, Razani-Boroujerdi S, Sopori ML. Nicotine inhibits Fc epsilon RI-induced cysteinyl leukotrienes and cytokine production without affecting mast cell degranulation through alpha 7/alpha 9/alpha 10-nicotinic receptors. J Immunol. 2010; 185:588–596.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download