Abstract
Allergic bronchopulmonary aspergillosis (ABPA) is a pulmonary disease with small prevalence. Exposure to aspergillus mold causes immunologic hypersensitivity and may cause ranges of symptoms from minimal to detrimental outcomes. Diagnosing and treating the disease before the development of bronchiectasis may save the patient from poor outcomes. This report presents a case of recurrent ABPA without any symptom of asthma, which impeded the correct diagnosis even after numerous hospitalizations.
Allergic bronchopulmonary aspergillosis (ABPA) is widely known as a disease caused by complex hypersensitivity reaction to bronchial colonization of Aspergillus fumigatus [1]. ABPA is a disorder that may develop bronchiectasis with severe, repetitive respiratory infection and it should be detected in the early stage before the disease develops to a more severe form with poorer outcome [2]. The symptoms of ABPA are also similar to other conditions of respiratory infections. Early and accurate diagnosis of ABPA may save the patients from unnecessary exposure to antibiotics.
It is commonly known that patients with baseline bronchial inflammatory diseases such as asthma are susceptible to ABPA [3]. History or symptoms of asthma are required in the current diagnostic criteria of ABPA [4]. Here, we report a case of recurrent ABPA with delayed diagnosis due to the absence of asthmatic symptoms.
A 71-year-old woman presented with a 1-week history of afebrile dyspnea with cough and sputum, tinged with small amount of blood. Her symptoms were concurrent with general weakness and myalgia. She was taking regular medications for her hypertension. Chest breathing sounds were clear without any wheezing or rales. The chest radiograph initially taken during the admission showed a consolidation in the right upper lung field (Fig. 1A). A chest computed tomography (CT) scan was taken for more specific visualization of the consolidation. The image showed a large consolidation (5.8 cm × 6.0 cm × 5.1 cm) with adjacent centrilobular opacities in the apical segment of right upper lung with central bronchiectasis and mucoid bronchial impaction. Secondary reactive lymph node hyperplasia was noted in the right hilum (1.2 cm) (Fig. 1B–D).
The past medical records of the patient were reviewed. She was never diagnosed with asthma nor was she recently complaining of asthmatic symptoms. She had been hospitalized four times previously and had visited the outpatient pulmonology clinic once under the impression of recurrent pneumonia. Her past chest radiographs and CTs revealed migrating consolidations at different sites, all during the repetitive admissions for her respiratory symptoms (Fig. 2). Empirical antibiotics and antiparasitic agents were given during these admission periods under the differential diagnosis of organizing pneumonia, paragonimiasis and lung cancer (Table 1). After each period of the treatment, the initial lesions had disappeared in the follow-up radiographic images. However, the patient did not show immediate alleviation of her symptoms after the initiation of the treatment. Rather, the symptoms seemed to alleviate after extended use of the antibiotics of more than 2 weeks. During these previous admissions, the patient received bronchoscopic biopsy for the upper respiratory symptoms that did not show immediate response after the use of antibiotics or antiparasites. The biopsy results showed dense inflammatory infiltration in the bronchial wall, composed of nonspecific lymphoplasma cells, eosinophils, and histoid cells (Fig. 3).
During the course of present admission, her complete blood cell counts were as follows: white blood cells, 8,990/mm3 (neutrophils, 60.1%; lymphocytes, 20.7%; monocytes, 5.8%; eosinophil, 13.0%; basophil, 0.4%); hemoglobin, 11.3 g/dL; and platelet, 302,000/mm3. Her eosinophil count was 1,170/mm3. The high sensitivity C-reactive protein was 33.55 mg/L and the procalcitonin was less than 0.05 ng/mL.
No pathogen was found in the blood and sputum cultures. Examinations were done to find tuberculosis, fungus, and parasites (including antibody studies for Cysticercus, Sparaganum, Paragonimus, Clonorchis, and Toxomplasma) but they all turned out to be negative. Serum total IgE level was 1,714 kU/L. Together with her eosinophilia, her disease entity pointed towards hypersensitivity reaction. Serum IgG and IgE antibodies specific to A. fumigatus were positive (13 U/mL and 5.45 kU/L, respectively). The intradermal test for A. fumigatus showed positive results (wheal of 16 cm × 9 cm and flare compared to saline). Pulmonary function test showed mildly restrictive pattern (forced vital capacity [FVC], 1.93 L [77% predicted]; forced expiratory volume in 1 second [FEV1], 1.42 L [77% predicted]; FEV1/FVC, 74%). However, the methacholine provocation test showed prominent airway hyperreactivity (PC20 = 4.6 mg/mL).
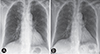
The patient was diagnosed with ABPA. The patient was started on oral prednisolone (40 mg/day) and oral itraconazole (200 mg/day). Chest radiographs were taken as follow-up studies during the medication. The initial consolidation showed immediate response to the treatment and decreased during the serial follow-up radiographic images. After subsequent 9 days of treatment with the 2 agents, she was discharged without any of her initial symptoms. One month later, the consolidation had disappeared completely (Fig. 4).
The pathogenesis of ABPA appears to be composed of complex hypersensitivity reactions, including types I, III, and IV hypersensitivity reactions with Aspergillus-specific IgE, IgG, and T-lymphocytes, respectively [5]. The complex mechanism behind the pathogenesis of ABPA presents with variety of symptoms from mild asthmatic symptoms to severe lung fibrosis.
The diagnosis of ABPA is difficult and suspecting the disease requires careful analyses of the patient's clinical presentations, including the clinical, laboratory, and radiographic findings. The patient in this case was hospitalized several times due to her respiratory symptoms and migrating consolidations in the serial chest radiographs. However, she had never been suspected of ABPA due to the lack of asthmatic symptoms. The diagnostic criteria of ABPA have recently been revised by the International Society for Human and Animal Mycology as the followings [4]: (1) either predisposing conditions of asthma or cystic fibrosis, (2) Aspergillus skin test positivity or detectable IgE levels against A. fumigatus, (3) elevated total serum IgE concentration (typically >1,000 IU/mL, but if the patient meets all other criteria, an IgE value <1,000 IU/mL may be acceptable), (4) at least 2 of the following conditions: precipitating serum antibodies to A. fumigatus, radiographic pulmonary opacities consistent with ABPA, or total eosinophil count >500 cells/µL in glucocorticoid-naive patients.
Past medical reports have shown that although rare, ABPA without asthma or cystic fibrosis can occur [6]. Agarwal et al. [7] did a systematic MEDLINE search of the literature using the free text term "allergic bronchopulmonary aspergillosis," and revealed 17 articles that have reported ABPA without any history or symptoms of asthma. This research provided 36 cases across the globe with 2 cases of bronchodilator reversibility [6] and 1 case of airway hyperresponsiveness to methacholine [8] as in this present case. Some patients may have airway hyperreactivity without asthmatic symptoms. This case shows that despite the current diagnostic criteria, patients without pre-existing asthmatic symptoms cannot completely be excluded from the possibility of ABPA.
The radiographic findings from the high-resolution computed tomography (HRCT) in this case are typical of ABPA. Central bronchiectasis (proximal cylindrical bronchiectasis with upper lobe predominance and bronchial wall thickening) affecting medial one-half to two-thirds of the lungs is considered a characteristic finding of ABPA, but has not been proved to be a sensitive or specific marker for diagnosing ABPA [910]. Additional findings of ABPA in HRCT include mucus plugging, atelectasis of dependent portion due to plugged mucus, high attenuated mucus, tree-in-bud appearance, and ground-glass opacities [11]. Such typical CT findings should arouse the physicians of the possibility of ABPA.
ABPA is still a disease entity hard to recognize due the similarities in symptoms and radiographic presentations with the more common disease such as pneumonia [112]. Conditions that fail to improve or recur after a course of antibiotics should arouse the physicians of ABPA. The antibiotics and antiparasitic agents that were used during the previous times of this patient did not show any therapeutic effect. The seemingly delayed response of the disease to the treatment agents was just a mere natural recovery from the hypersensitivity reaction.
Although no clinical trials have been done, the key treatment of ABPA is considered to be corticosteroids. Corticosteroids reduce the inflammation induced by the immunological responses and usually lead to dramatic clinical improvement. A case series of 126 ABPA patients treated with corticosteroids has been reported. All patients developed remission of the disease with more than 35 percent in decline of IgE levels and clearance of chest radiographic lesions by 6 weeks [13].
Antifungal agents are considered in the treatment scheme of ABPA for the reduction of the antigenic stimulus for bronchial inflammation. The addition of itraconazole may reduce the long term period of corticosteroid treatment [14]. Voriconazole has been noticed recently as another successful antifungal agent for treating ABPA [15]. However, comparison between the 2 agents has not been done and the superiority of voriconazole has not yet been proven.
ABPA has a good prognosis if diagnosed and treated in the early stage. Suspecting the disease in its earlier course is crucial for avoiding unnecessary antibiotics and preventing severe lung destruction that may occur in the advanced course of the disease. The absence of asthma history may be a misleading factor for correct diagnosis. Clinical suspicion for ABPA is necessary when patients with lung infiltration with high serum total IgE do not respond to antibiotics.
Figures and Tables
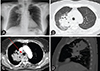 | Fig. 1The radiographic findings during the current admission (August, 2015). The isolated, large consolidation (5.8 cm × 6.0 cm × 5.1 cm) with central bronchiectasis and mucoid bronchial impaction is found in the right upper lobe of lung parenchyme. (A) Chest simple radiograph, (B) cross section of the consolidation in high resolution chest computed tomography (CT), (C). cross section of the consolidation in enhanced chest CT (arrow: a reactive peribronchial lymph node enlargement, 1.2 cm), (D) sagittal section of the consolidation in enhanced chest CT. |
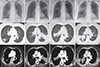 | Fig. 2The radiologic findings during the past admissions. Past computed tomography (CT) findings reveal migrating consolidations all at different locations of lung. All CTs were taken during which the patient presented with respiratory symptoms. The patient presented with (A) consolidation in right lower lobe (November, 2011, event 1), (B) larger consolidation in left lower lobe (March, 2013, event 2), (C) consolidation in posterior part of right upper lobe (September, 2013. event 3), and (D) larger consolidation in posterior part of right upper lobe (November, 2013, event 4). |
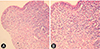 | Fig. 3(A) The bronchoscopic biopsy shows dense inflammatory infiltration in the bronchial wall (H&E, ×200). (B) The inflammatory cells are nonspecific, composed of lymphplasma cells, eosinophils, and histioid cells (H&E, ×400). |
References
1. Greenberger PA. Allergic bronchopulmonary aspergillosis. J Allergy Clin Immunol. 2002; 110:685–692.

3. Riscili BP, Wood KL. Noninvasive pulmonary Aspergillus infections. Clin Chest Med. 2009; 30:315–335.

4. Agarwal R, Chakrabarti A, Shah A, Gupta D, Meis JF, Guleria R, Moss R, Denning DW. ABPA complicating asthma ISHAM working group. Allergic bronchopulmonary aspergillosis: review of literature and proposal of new diagnostic and classification criteria. Clin Exp Allergy. 2013; 43:850–873.

5. Wang JL, Patterson R, Rosenberg M, Roberts M, Cooper BJ. Serum IgE and IgG antibody activity against Aspergillus fumigatus as a diagnostic aid in allergic bronchopulmonary aspergillosis. Am Rev Respir Dis. 1978; 117:917–927.
6. Glancy JJ, Elder JL, McAleer R. Allergic bronchopulmonary fungal disease without clinical asthma. Thorax. 1981; 36:345–349.

7. Agarwal R, Aggarwal AN, Gupta D, Bal A, Das A. Case report: a rare cause of miliary nodules -- allergic bronchopulmonary aspergillosis. Br J Radiol. 2009; 82:e151–e154.
8. Yoshida N, Suguro H, Kohara F, Akiyama Y, Katoh H, Hashimoto N, Majima T, Yamaguchi M, Horie T, Kawabata Y. A case of allergic bronchopulmonary aspergillosis with no history of bronchial asthma. Nihon Kyobu Shikkan Gakkai Zasshi. 1992; 30:2123–2127.
9. Reiff DB, Wells AU, Carr DH, Cole PJ, Hansell DM. CT findings in bronchiectasis: limited value in distinguishing between idiopathic and specific types. AJR Am J Roentgenol. 1995; 165:261–267.

10. Ward S, Heyneman L, Lee MJ, Leung AN, Hansell DM, Muller NL. Accuracy of CT in the diagnosis of allergic bronchopulmonary aspergillosis in asthmatic patients. AJR Am J Roentgenol. 1999; 173:937–942.

11. Agarwal R, Gupta D, Aggarwal AN, Saxena AK, Chakrabarti A, Jindal SK. Clinical significance of hyperattenuating mucoid impaction in allergic bronchopulmonary aspergillosis: an analysis of 155 patients. Chest. 2007; 132:1183–1190.
12. D'Urzo AD, McIvor AR. Case report: allergic bronchopulmonary aspergillosis in asthma. Can Fam Physician. 2000; 46:882–884.
13. Agarwal R, Gupta D, Aggarwal AN, Behera D, Jindal SK. Allergic bronchopulmonary aspergillosis: lessons from 126 patients attending a chest clinic in north India. Chest. 2006; 130:442–448.
14. Walsh TJ, Anaissie EJ, Denning DW, Herbrecht R, Kontoyiannis DP, Marr KA, Morrison VA, Segal BH, Steinbach WJ, Stevens DA, van Burik JA, Wingard JR, Patterson TF. Infectious Diseases Society of America. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis. 2008; 46:327–360.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download