Abstract
Background
Asthma patients may experience acute episodic exacerbation. The guidelines recommend that written action plan should be given to asthma patients. However, no one can predict when and where acute exacerbation will happen. As people carry smart phone almost anytime and anywhere, smartphone application could be a useful tool in asthma care. We evaluated the feasibility of the ubiquitous healthcare system of asthma care using a smartphone application (snuCare) based on the self-management guideline or action plan.
Methods
Forty-four patients including fragile asthmatics were enrolled from Seoul National University Bundang Hospital between December 2011 and February 2012. They were randomly assigned into application user (n = 22) or application nonuser group (n = 22). We evaluated user-satisfaction, and clinical parameters such as asthma control, Quality of Life Questionnaire for Adult Korean Asthmatics, and the adherence of patients.
Results
The characteristics were similar at baseline between the 2 groups except those who treated with short-term systemic steroid or increased dose of systemic steroid during previous 8 weeks (user vs. nonuser: 31.8% vs. 4.5%, p = 0.020). Total of 2,226 signals was generated during 8 weeks including 5 risky states. After eight weeks, the users answered that it was very easy to use the application, which was shown in highest scores in terms of satisfaction (mean ± standard deviation, 4.3 ± 0.56). Seventy-three percent of patients answered that the application was very useful for asthma care. User group showed improved the adherence scores (p = 0.017). One patient in application user group could avoid Emergency Department visit owing to the application while a patient in nonuser group visited Emergency Department.
Asthma is a dynamic disease characterized by acute exacerbation. The asthma exacerbation usually results from inadequate asthma control, and leads to major asthma morbidity and mortality [1]. Therefore, international guidelines indicate that the primary goal of asthma management is to achieve optimal control [23]. At the same time, the guidelines have recommended that all asthmatic patients receive a written asthma action plan [3], which facilitates the early detection and self-management of asthma exacerbation [4].
However, it is not clear that the written asthma action plan fitted well into the real practice. The utilization may be frequently hindered by poor user-friendliness or compatibility [5]. In this regard, we have postulated that a smartphone application may help patients to follow asthma action plan, improve self-management and finally prevent asthma exacerbation. Several previous studies have explored the feasibility and efficacy of electronic device-assisted asthma self-management systems [678]; however, none in the literature still evaluated the telemedicine system which interacts with asthma specialist care in real time. We have developed a smartphone based asthma care system (snuCare, Division of Allergy and Clinical Immunology, Seoul National University Bundang Hospital, Seongnam, Korea) which includes an interactive asthma action plan based on the self-management guideline. Here we aimed to explore the feasibility and efficacy of the snuCare in adult asthma patients visiting an allergic clinic in a University hospital.
We prospectively enrolled 44 patients including fragile asthmatics who were diagnosed as asthma by physician and over 19 years old from Seoul National University Bundang Hospital between December 2011 and February 2012. They were randomly assigned into a smartphone application user group (n = 22) or a control (nonuser) group (n = 22). During recruitment, subjects were not included if they were not familiar with using smartphone or not willing to use a smartphone application. The study period was 8 weeks consisting of 3 visits (visit 0, baseline visit; visit 1, 4 weeks; and visit 2, 8 weeks). At baseline (visit 0), they were evaluated for demographic and clinical characteristics, pulmonary function test, asthma control test (ACT) [9], adherence of medication by self-assessment from 0 to 100 score, and Quality of Life Questionnaire for Adult Korean Asthmatics (QLQAKA) [10]. At visit 1, they were followed up for pulmonary function test, ACT and adherence of medication; and at visit 2, they were re-evaluated for pulmonary function test, ACT, adherence of medication, QLQAKA, and satisfaction questionnaire for the application. This study was approved by the Institutional Review Board of Seoul National University Bundang Hospital, Seongnam, Korea.
The user group was provided the snuCare application and peak flow meter, and was instructed for how to use in details before the clinical trials. The application was developed on the basis of a written asthma action plan [111]. It included the questionnaire items from the action plan, and let the patients to input their daily symptom score. The participants were instructed to record their asthma symptoms and peak expiratory flow (PEF) twice a day regularly, and also were asked to record the symptoms and PEF when their asthma worsened. The application followed the logic of the written asthma action plan, and was set to give daily signals to the users about their asthma control status, such as 'doing well,' 'getting worse,' 'medical alert,' or 'emergent situation,' based on symptoms and PEF values, and offered action plans to patients. All these input were sent to the online server in a real time manner, and risky signals such as 'medical alert' or 'emergent situation' were also sent to the corresponding researchers by short-message service. The online server was programmed to send clinical information relevant to asthma symptoms and PEF, based on the written asthma action plan. The researchers reviewed the electronic input from the patients when risky signals were generated, and made direct calls to the patients for assisting their self-management.
Statistical analyses were performed with IBM SPSS ver. 18.0 (IBM Co., Armonk, NY, USA). Continuous or categorical variables were presented as median and range in parentheses (mean ± standard deviation may be better for providing information, despite small sample size), or frequency and percentage in parentheses, respectively. Comparisons between groups were made by nonparametric test (two related samples). A p value was considered as significant if two-sided p < 0.05.
A total of 44 patients were enrolled in this study. Baseline characteristics were summarized in Table 1. There was no statistically significant difference between the 2 groups in their demographic characteristics except those who treated with short-term systemic steroid or increased dose of systemic steroid during previous 8 weeks (user vs. nonuser: 31.8% vs. 4.5%, p = 0.020) and those who treated with short-term systemic steroid or increased dose of systemic steroid during previous 1 year (user vs. nonuser: 59.1% vs. 27.3%, p = 0.035). About 15% of participants had previously visited Emergency Department due to asthma exacerbation, and none had been hospitalized for asthma within the previous year. Baseline asthma outcomes such as PFT, ACT, adherence, or QLQAKA scores also did not differ between the 2 groups.
Feasibility of using the asthma application were evaluated in the user group (n = 22, Fig. 1). A total of 2,226 signals were generated by 22 patients during 8-week trials (average 1.8 signal generation per day in each patient), indicating a fair adherence to the snuCare program (Fig. 2)
User satisfaction was asked for 17 questions from 6 categories; the satisfaction scores were faire (average 4 of 5). The score was the highest on the item 'ease to use' (4.3 ± 0.6). Scores on accuracy, form, overall satisfaction and speed were next in order (4.1 ± 0.5, 4.0 ± 0.9, 4.0 ± 0.6, and 4.0 ± 0.5). At visit 2, we asked patients that the application was helpful to control asthma in user group. Fourteen patients (64%) answered that application was moderately helpful. Numbers of patients who answered that application was very helpful were 2 (9%) and 5 patients (23%) answered that application was somewhat helpful.
During the 8-week follow-ups, several outcomes were evaluated for asthma. Lung function parameters, particularly forced expiratory volume in 1 second (FEV1), did not significantly differ between visits, or between the 2 groups at each visit. Changes of pulmonary function, asthma control status, drug compliance and quality of life in asthmatics were presented in Fig. 3 and Table 2. At baseline, FEV1 was 93% of predicted value as median in user group, and 91% of predicted value as median in nonuser group. After 8 weeks (visit 2), FEV1 was changed to 90% in user group and 100% in nonuser group. But, changes of FEV1 in both groups were not significant (p = 0.277 in user group vs. p = 0.217 in nonuser group). ACT was 22 points in both groups at baseline. After 8 weeks, ACT was changed to 21 points in user group, and 23 points in nonuser group. Changes of asthma control status according to ACT scores were not significant in both groups (p = 0.920 in user group vs. p = 0.571 in nonuser group).
The adherence was similar in both groups at baseline. However, the adherence became better in user group compared with nonuser group (p = 0.017 in user group vs. p = 0.674 in nonuser group). At baseline, QLQAKA was 67 points as median value in user group, and 69 points as median in nonuser group. At visit 2, QLQAKA was changed to 70 points in user group and 72 points in nonuser group. Changes of QLQAKA in user group were significant (p = 0.027), but were not significant in nonuser group (p = 0.139).
During the study, 1 patient in application user group could avoid Emergency Department visit owing to the application while a patient in nonuser group visited Emergency Department.
The number of patients who were treated with short-term systemic steroid or increased dose of systemic steroid during use of application (8 weeks) was similar between both groups (user vs. nonuser: 5 patients vs. 3 patients (21.7% vs. 13.6%), p = 0.440). It was an improved result from the baseline as described earlier (user vs. nonuser, 7 patients vs. 1 patient (31.8% vs. 4.5%), p = 0.020).
This study showed that a smartphone application (snuCare) was feasible for the monitoring and the management of asthma based on the self-management guideline. It is remarkable that the smartphone application (snuCare) could enhance the adherence of medications in asthmatics in this study. Many applications that assist patients to control asthma have been developed. However, the evaluation of feasibility is still insufficient. In a recent study, researchers investigated whether 103 applications for asthma in English provide evidence based information and applications support to self-management or not [12]. Almost applications provided inaccurate or unsafe information and only 32 applications provided strategies for asthma control [12]. In 2013, Licskai et al. [13] reported the pilot study about asthma action plan smartphone application and suggested its feasibility. In that study, there was significant improvement of asthma quality of life questionnaire score and most subjects (95%, the number of total users = 22) desired to use application continuously after the study [13]. Recently, Cook et al, [14] reported the efficacy of improving asthma control using smartphone application as a proof of concept study. Although, the application was designed simply without requiring regular input, this application could improve asthma control in the patients with uncontrolled asthma by providing individualized teaching and treatment support. In adolescents, the feasibility and utilization of smartphone application to control asthma [15].
We evaluated the feasibility of our asthma application by satisfaction survey to users in Korea. In addition, changes of ACT, QLQAKA scores, and FEV1 values were evaluated. Overall, the patients showed a high level of satisfaction with the application. Especially, scores about the form including ease to understand, convenience and ease to use was over 4.0 points. These are very encouraging results. Although the mean age of user group was 49, 72-year-old patient was included and he gave 5, 5 and 4 points to the form. Elderly patients who are not feasible to visit the hospital can be easily assisted to control asthma symptoms by application based on the self-management guideline if they could be trained to use it appropriately.
Eight weeks maybe too short to evaluate significant clinical improvement. There were no significant differences in changes of FEV1 and ACT after use the application. However, during the use of the application, the number of patients who were treated with short-term systemic steroid or increased dose of systemic steroid became similar (user vs. nonuser: 5 patients vs. 3 patients (21.7% vs. 13.6%), p = 0.440) while there was a significant difference in the number at baseline (user vs. nonuser: 7 patients vs. 1 patient [31.8% vs. 4.5%], p = 0.020).
It was also remarkable that one patient in application user group could avoid Emergency Department visit owing to the application while a patient in nonuser group visited Emergency Department.
In this study, QLQAKA scores in nonuser group were increased after 8 weeks. We did not expect this result but it could be due to the baseline characteristics. The number of patients who were administered systemic steroid before the study (recent 8 weeks and remote 1 year) in user group was significantly higher than in nonuser group. We assumed that this difference might affect the result.
There are limitations in our study. This was a preliminary study with 44 patients for 8 weeks to check the feasibility. This study showed that the asthma application was feasible and could be useful especially for the adherence, the prevention of visiting Emergency Department and the reduction of systemic steroid. This suggested that the ubiquitous healthcare system using a smartphone application (snuCare) based on the self-management guideline could be helpful in the monitoring and the management of asthma.
Figures and Tables
 | Fig. 1(A) The questionnares on the satisfaction using the smartphone application. (B) Most of the users anwered that the smartphone application (snuCare, Division of Allergy and Clinical Immunology, Seoul National University Bundang Hospital, Seongnam, Korea) based on the self-management guideline was helpful to control asthma. |
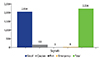 | Fig. 2Signal generated by applications; Mean number of signal per each person ± standard deviation = 101 ± 26.9. |
 | Fig. 3Changes of FEV1, ACT, adherence of medication and QLQAKA. (A–D) Application users; (E–H) Application nonusers. Values are presented as median (range). FEV1, forced expiratory volume in 1 second; ACT, asthma control test; QLQAKA, Quality of Life Questionnaire for Adult Korean Asthmatics; V1, 4-week study period; V2, 8-week study period. *p value, statistical method was the nonparametric test. |
ACKNOWLEDGEMENTS
This work was supported by the KT-Seoul National University Bundang Hospital (SNUBH) Collaborative Research Fund.
References
1. Bateman ED, Hurd SS, Barnes PJ, Bousquet J, Drazen JM, FitzGerald M, Gibson P, Ohta K, O'Byrne P, Pedersen SE, Pizzichini E, Sullivan SD, Wenzel SE, Zar HJ. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J. 2008; 31:143–178.

2. Levy ML, Thomas M, Small I, Pearce L, Pinnock H, Stephenson P. Summary of the 2008 BTS/SIGN British Guideline on the management of asthma. Prim Care Respir J. 2009; 18:Suppl 1. S1–S16.

3. Urbano FL. Review of the NAEPP 2007 expert panel report (EPR-3) on asthma diagnosis and treatment guidelines. J Manag Care Pharm. 2008; 14:41–49.

4. Gibson PG, Powell H, Coughlan J, Wilson AJ, Abramson M, Haywood P, Bauman A, Hensley MJ, Walters EH. Self-management education and regular practitioner review for adults with asthma. Cochrane Database Syst Rev. 2003; (1):CD001117.

5. Ring N, Jepson R, Hoskins G, Wilson C, Pinnock H, Sheikh A, Wyke S. Understanding what helps or hinders asthma action plan use: a systematic review and synthesis of the qualitative literature. Patient Educ Couns. 2011; 85:e131–e143.

6. Lee HR, Yoo SK, Jung SM, Kwon NY, Hong CS. A Web-based mobile asthma management system. J Telemed Telecare. 2005; 11:Suppl 1. 56–59.

7. Holtz B, Whitten P. Managing asthma with mobile phones: a feasibility study. Telemed J E Health. 2009; 15:907–909.

8. Marcano Belisario JS, Huckvale K, Greenfield G, Car J, Gunn LH. Smartphone and tablet self management apps for asthma. Cochrane Database Syst Rev. 2013; (11):CD010013.

9. Kwon HS, Lee SH, Yang MS, Lee SM, Kim SH, Kim DI, Sohn SW, Park CH, Park HW, Kim SS, Cho SH, Min KU, Kim YY, Chang YS. Correlation between the Korean version of Asthma Control Test and health-related quality of life in adult asthmatics. J Korean Med Sci. 2008; 23:621–627.

10. Park JW, Cho YS, Lee SY, Nahm DH, Kim YK, Kim DK, Sohn JW, Park JK, Jee YK, Cho YJ, Yoon HJ, Kim MK, Park HS, Choi BW, Choi IS, Park CS, Min KU, Moon HB, Park SH, Lee YK, Kim NS, Hong CS. Multi-center study for the utilization of quality of life questionnaire for adult Korean asthmatics (QLQAKA). J Asthma Allergy Clin Immunol. 2000; 20:467–480.
11. The Korean Academy of Asthma, Allergy, and Clinical Immunology. Korean asthma management guideline. 2011 [Internet]. Seoul: The Korean Academy of Asthma, Allergy, and Clinical Immunology;c2005-2016. cited 2016 Feb 18. Available from http://www.allergy.or.kr/public/March_2011.pdf.
12. Huckvale K, Car M, Morrison C, Car J. Apps for asthma self-management: a systematic assessment of content and tools. BMC Med. 2012; 10:144.

13. Licskai C, Sands TW, Ferrone M. Development and pilot testing of a mobile health solution for asthma self-management: asthma action plan smartphone application pilot study. Can Respir J. 2013; 20:301–306.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download