Abstract
Background
Although many patients with allergic rhinitis have symptoms due to sensitization to more than one kind of allergens, and mixed allergen extracts are widely used for immunotherapy, there are few published trials.
Objective
Our study aimed to evaluate the effect of multiple-allergen immunotherapy on improving the symptoms and quality of life of allergic rhinitis patients.
Methods
We performed a 1-year single-center observation study of subcutaneous immunotherapy using house dust mite extract (n = 12), weed pollen extract (n = 21), or mixed house dust mite/weed pollen extract (n = 11) in 44 allergic rhinitis patients. All the allergens responsible for the symptom of each patient were included in his immunotherapy. Symptom score, medication score, and quality of life of the patients were evaluated before and after 1-year immunotherapy. Quality of life was evaluated with the Rhinoconjunctivitis Quality of Life Questionnaire.
Results
In all 3 groups receiving subcutaneous immunotherapy, significant improvement of symptom score, medication score, and quality of life was found vs. baseline at 1 year, irrespective of the allergen used. In the weed pollen season, the changes of quality of life questionnaire score after 1-year treatment were not significantly different between the weed pollen group (1.55 ± 1.24) and the mixed house dust mite/weed pollen group (1.14 ± 1.01). The same happened in the nonpollen seasons, during which dust mite immunotherapy (1.23 ± 1.63) and mixed immunotherapy (0.60 ± 0.47) did not show significantly different effect on the quality of life.
Allergen immunotherapy has been proven to be an effective treatment for both allergic rhinitis/asthma by a lot of clinical trials. Most of these studies employed only a single allergen extract to treat the patients, and it has been claimed that allergen immunotherapy is especially effective in monosensitized individuals [12]. But most of atopic patients suffering from allergic rhinitis/asthma are usually polysensitized, who produce specific IgE against different allergens and may need immunotherapy including multiple allergens [3]. So far it is still controversial whether the efficacy of mono-allergen immunotherapy can be extended to mixtures of multiple allergen extracts, and clinical evidences supporting multiple-allergen immunotherapy are lacking. Our study aimed to investigate the effect of multiple-allergen immunotherapy on improving the symptoms and quality of life of allergic rhinitis patients.
This was a single-center observation study performed over 1 year, including 44 adult patients living in Beijing, with moderate to severe persistent allergic rhinoconjunctivitis due to sensitization to house dust mite, or weed pollens, or both of them. The patients should have positive (≥++) intradermal tests (IDTs) to house dust mite, and/or at least one kind of common weed pollens in Northern China, including Mugwort, Humulus, and Kochiascoparia pollens (Xieh eXinhualian Pharmacy, Beijing, China). The result of IDT was defined according to the mean diameter of the wheal: (+) was defined as a wheal 5–9 mm at 15 minutes, (++) represented a wheal 10–15 mm, (+++) was a wheal 16–19 mm, and (++++) was a wheal ≥20 mm. All the wheals should be accompanied by itching and surrounding flares. Also serum specific IgE against house dust mite, and/or weed pollens of ≥class 2 (ImmunoCAP, Phadia, Sweden) was required. In addition, patients had to report symptoms compatible with sensitization to house dust mite, and/or weed pollens. Those patients allergic to other aeroallergens or having coexisting asthma were excluded. This study was approved by Peking Union Medical College Hospital Ethics Committees, and all patients gave written informed consent.
All the allergic rhinitis patients were recruited in 2013, and were allocated to receive subcutaneous immunotherapy (SCIT) after a baseline evaluation. As displayed in Fig. 1, all the SCIT procedures started in December, which was 2 months after the weed pollen season in North China, and lasted for the next whole year. According to the aeroallergens to which our patients were sensitized, they were divided into 3 groups. Dust mite group (n = 12) was allergic to house dust mite and received SCIT for dust mite. Weed pollen group (n = 21) allergic to weed pollens was given SCIT for corresponding pollens. Mixed group (n = 11) was sensitized to both dust mite and weed pollens, so that SCIT with mixed dust mite-weed pollen extract was prescribed.
The highest concentration of house dust mite extract was w/v 1:20, and the highest concentration of each kind of weed pollen extract applied in this study was w/v 1:25 (XieheXinhualian Pharmacy). The allergen extract used in the maintenance therapy was 1:10 dilution of its highest concentration. For those patients who needed multiple-allergen immunotherapy, their maintaining treatment mixtures were prepared by combining 1 mL of the highest concentration available for each treatment allergen with albumin-saline diluent to a total volume of 10 mL per treatment vial.
The build-up phase of SCIT was initiated with 0.1 mL of a 1:1,000 dilution of maintenance concentrate, and injections were increased weekly to reach a target maintenance dose of 1.0 mL of concentrate. Maintenance therapy was given once every week for 12 months.
All patients were prescribed rescue medication: oral antihistamine (loratadine), nasal antihistamine (azelastine) and nasal corticosteroid (mometasone), which can be used on demand.
Pollen counts were performed daily with the method of gravity sedimentation in Allergy Department of Peking Union Medical College Hospital in Beijing, China. The mean weekly count of the grains per cubic meter was calculated.
Daily symptom score (SS): the symptoms of nose (sneezing, itching, rhinorrhea, stuffy nose) and eye (itching, redness, swelling, watery eye) were scored on a scale ranging from 0 to 3 (0 = none; 1 = slight, the symptom is clearly present but is not troublesome; 2 = moderate, the symptom is present, it is troublesome, but not disabling or insufferable; and 3 = severe, the symptom is severe, disabling and/or insufferable). The daily SS was calculated as the sum of all individual scores related to nose and eye.
Daily medication score (MS): the intake of medication was also recorded in the same day and was quantified according to the rule described in Table 1.
As showed in Fig. 1, the patients of dust mite group recorded their daily SS and MS once per week in November, 2013. The mean of these daily scores was registered as the baseline SS or MS. Following the same rule, weed pollen group recorded SS and MS in the weed pollen season (usually in August and September), and mixed group evaluated SS and MS both in the weed pollen season and in November. The same pattern of evaluation was repeated by all the three groups in 2014, therefore SS and MS after 1-year immunotherapy could be quantified.
The standardized Rhinoconjunctivitis Quality Of Life Questionnaire (RQLQ) developed by Juniper et al. [4] was used. RQLQ contains 28 questions which cover 7 domains: activities, sleep, nonhay fever symptoms, practical problems, nasal symptoms, eye symptoms and emotion. Patients rated each item on a scale of 0 (not troubled) to 6 (extremely troubled). The scores of the domains were expressed as the mean score for each item. The overall RQLQ was expressed as the mean of 7 domain scores. Patients completed the questionnaire at the same time when they record their SS and MS, both at the baseline and after 1-year SCIT.
Comparison of sex ratio at baseline was tested by Pearson chi-square, and analysis of variance test was used to verify the difference of patient age among 3 groups. The paired-samples t test was used to compare the SS, MS, and RQLQ scores before and after 1-year immunotherapy. The changes of RQLQ in different therapy groups were compared using the Wilcoxon rank sum test. A p < 0.05 was considered significant. All the statistical analyses have been computed using IBM SPSS Statistics ver. 19.0 (IBM Co., Armonk, NY, USA).
Forty-four adult patients (mean age, 34 years; age range, 20–54 years; 21 men) were enrolled and allocated to 3 groups according to the allergens used in their immunotherapy: the house dust mite group (n = 12), the weed pollen group (n = 21), and the mixed house dust mite/weed pollen group (n = 11). Age and sex ratio did not significantly differ in the three groups as indicated in Table 2.
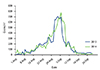
The weed pollen counts in the pollen season in 2013 and 2014 were illustrated in Fig. 2. The 2 monitored weed pollen seasons had a similar global pollen load.
In all 3 groups receiving SCIT, a significant clinical improvement was found vs. baseline at 1 year, irrespective of the allergen, as showed in Fig. 3. After 1-year immunotherapy, SS, MS, and RQLQ score of the dust mite group were all significantly lower than baseline scores (p < 0.05). SS, MS, and RQLQ score in the weed pollen season of the weed pollen group were also significantly better than baseline level (p < 0.01). As to the mixed house dust mite/weed pollen group, SS, MS, and RQLQ score both in the weed pollen season and in the nonpollen season (November) decreased significantly compared to those scores evaluated 1 year before (p < 0.01).
In the weed pollen season, the changes of RQLQ score after 1-year treatment were not significantly different between the weed pollen group (1.55 ± 1.24) and the mixed house dust mite/weed pollen group (1.14 ± 1.01). The same happened in the nonpollen seasons, during which dust mite SCIT (1.23 ± 1.63) and mixed SCIT (0.60 ± 0.47) did not show significantly different effect on the quality of life.
SCIT was well tolerated by the patients, and all of them reached the maximum dose planned. Two patients in the weed pollen group and 1 patient in the mixed dust mite/weed pollen group experienced local reactions and there were no systemic reaction among patients from all the groups during the study.
Respiratory allergy is often characterized by the presence of multiple sensitizations, with only approximately 20% of patients being monosensitized [2]. On the other hand, most clinical trials assessing the efficacy of immunotherapy have been performed in the monosensitized group with a single allergen. What kind of strategy should be adopted in the immunotherapy of the polysensitized patients, or whether multiple-allergen immunotherapy is effective in these patients is still a matter of debate [3].
Some authors claim that the multiple-allergen immunotherapy with mixed allergens have two deficiencies. Firstly, the additional extracts could dilute the preexisting allergens; secondly, there might be deleterious effect of one extract upon another in the mixture [5]. In fact, as long as more attentions are paid to the preparation of the multiple-allergen extracts, these problems could be solved properly. In order to make for the first deficiency, larger amount of each stock extract should be added into the final mixture to make sure that the effective dose of every allergen is achieved in the final treatment set of vaccine [6]. As to the second issue, the degradation of one extract by another could also be avoided by placing the allergens with strong proteases in a separate vial and using several vials respectively [7].
In order to prove the effectiveness of multiple-allergen immunotherapy in clinical practice, several clinical studies with different levels of quality have been conducted. However, these researches have got conflicting results.
Some papers showed that multiple-allergen therapy had no better effect than the placebo. Bousquet et al. [8] conducted a SCIT study on 70 adults with allergic rhinitis (AR) sensitized to orchard grass alone or orchard grass plus other allergens. Patients sensitized to orchard grass alone received either orchard grass SCIT or placebo, while polysensitized patients received SCIT with orchard grass and other extracts they were sensitized to, or placebo. Clinical improvement was only detected in those receiving single-allergen SCIT, and no benefit over placebo was detected in patients receiving multi-allergen SCIT [8]. Also In Adkinson's study, 121 children with allergic asthma were randomized to receive either placebo or up to 7 extracts (including house dust mite, grass, Alternaria, Aspergillus, and Cladosporium), and after 30 months of treatment, there was no significant difference between SCIT and placebo groups based on symptom MSs, and peak expiratory flow values. The author pointed out that this result was might because all the patients in this study had good compliance and received intensive pharmacologic therapy, with frequent follow-up visit. All these measures might have controlled the disease process to such an extent that it would have been difficult to show the clinical benefit of immunotherapy [9].
Yet other studies had different conclusions. In a SCIT study included 53 adults with seasonal AR and/or asthma sensitized to orchard grass and olive tree pollens. Patients received either a mixture of orchard grass and olive tree pollens or placebo. After 1-year treatment, symptom, medication and quality of life assessment scores were significantly lower in the active group [10]. In another study, 54 children with allergic rhinoconjuctivitis were treated with a mixture of up to 4 allergens including animal dander, dust mite and cockroach. After 6 months, immunotherapy also showed better effect than pharmacotherapy [11].
In our study, the paired-samples test showed that the SSs, MSs, and RQLQs in both the weed pollen season and ordinary times (nonpollen seasons) decreased significantly after 1-year SCIT using mixed dust mite/weed pollens extract, compared to baseline levels in the patients who sensitized to both dust mite and weed pollens. This result suggested that multiple-allergen immunotherapy could effectively improve the symptoms and quality of life of the allergic rhinitis patients. Due to lack of funds and shortage of manpower, our study did not include a placebo group. If we had the placebo group as a control, the evidence would be much more convincing.
On the other hand, we compared the changes of RQLQ scores recorded in ordinary times between the dust mite group and mixed dust mite/weed pollens group, and found that the diminutions were not significantly different between the 2 groups. The same condition happened in the weed pollen season, during which weed pollens SCIT and mixed SCIT did not show significantly different effect. Although we did not find different benefit on quality of life between multiple-allergen and mono-allergen immunotherapy, yet these negative results might be due to the small sample size of our study, and we still could not conclude that the effect was equivalent while the dust mite allergen was used alone or while it was mixed with other unrelated allergens.
Because of the ethical requirements, in our study all the allergens responsible for the symptom of each patient had to be included in his immunotherapy, and we could not use only dust mite or only weed pollen extract to treat patients sensitized to both of them. If we could recruit patients sensitized to both dust mite and pollen, and divide them into 4 groups, who receive SCIT for dust mite, SCIT for pollen, SCIT for dust mite and pollen, or placebo respectively, we would obtain more accurate information about the performance of single versus multiple unrelated allergens immunotherapy.
In summary, the multiple-allergen immunotherapy might be effective in polysensitized populations. It could improve symptoms and quality of life in allergic rhinitis patients. Our result did not show significant difference between the effects of multiple-allergen immunotherapy and mono-allergen immunotherapy. But the experimental evidences are still weak, and more clinical trials with larger sample size and higher quality are needed to draw a convincing conclusion.
Figures and Tables
 | Fig. 1Study design. Asterisk (*) means the patients recorded their daily symptom scores and medication scores once per week, and simultaneously evaluate their scores of Rhinoconjunctivitis Quality of Life Questionnaire every week in this month. |
 | Fig. 3Symptom scores, medication scores, and quality of life scores of patients in different grouos before and after the 1-year subcutaneous immunotherapy. The efficacy of immunotherapy in the mixed dust mite/weed pollen group was evaluated both in the weed pollen season and nonpollen seasons (ordinary time). (A) Symptom score, (B) medication score, and (C) Rhinoconjunctivitis Quality of Life Questionnaire score (RQLQS). |
References
1. Bousquet J, Lockey R, Malling HJ. Allergen immunotherapy: therapeutical vaccines for allergic diseases. A WHO position paper. J Allergy Clin Immunol. 1998; 102:558–562.
2. Li Q, Li M, Yue W, Zhou J, Li R, Lin J, Li Y. Predictive factors for clinical response to allergy immunotherapy in children with asthma and rhinitis. Int Arch Allergy Immunol. 2014; 164:210–217.

3. Bousquet PJ, Castelli C, Daures JP, Heinrich J, Hooper R, Sunyer J, Wjst M, Jarvis D, Burney P. Assessment of allergen sensitization in a general population-based survey (European Community Respiratory Health Survey I). Ann Epidemiol. 2010; 20:797–803.

4. Juniper EF, Thompson AK, Ferrie PJ, Roberts JN. Validation of the standardized version of the Rhinoconjunctivitis Quality of Life Questionnaire. J Allergy Clin Immunol. 1999; 104(2 Pt 1):364–369.

5. Nelson HS. Multiallergen immunotherapy for allergic rhinitis and asthma. J Allergy Clin Immunol. 2009; 123:763–769.

6. Nelson HS, Iklé D, Buchmeier A. Studies of allergen extract stability: the effects of dilution and mixing. J Allergy Clin Immunol. 1996; 98:382–388.

7. Nelson HS. Specific immunotherapy with allergen mixes: what is the evidence? Curr Opin Allergy Clin Immunol. 2009; 9:549–553.

8. Esch RE. Allergen immunotherapy: what can and cannot be mixed? J Allergy Clin Immunol. 2008; 122:659–660.

9. Bousquet J, Becker WM, Hejjaoui A, Chanal I, Lebel B, Dhivert H, Michel FB. Differences in clinical and immunologic reactivity of patients allergic to grass pollens and to multiple-pollen species. II. Efficacy of a double-blind, placebo-controlled, specific immunotherapy with standardized extracts. J Allergy Clin Immunol. 1991; 88:43–53.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download