Abstract
Background
The new International Classification of Diseases (ICD)-11 "Allergic and hypersensitivity conditions" section has been constructed as a result of a detailed and careful action plan based on scientific evidences for the necessity of changes and collaboration with the World Health Organization (WHO) ICD-11 revision governance. All the efforts are being acknowledged by the Joint Allergy Academies.
Objective
Considering the new classification model addressed to the allergic and hypersensitivity conditions and following the ICD WHO agenda, we believe it is the appropriate time to start supporting the validation process in collaboration with the WHO ICD governance.
Methods
We conducted a mapping of ICD-10 allergic and hypersensitivity conditions in the ICD-11 beta phase structure and categorized the conditions as fitting by "precoordination," "postcoordination," "indexed to the ICD-11 Foundation," "no code fit properly" or "no correspondence" in the ICD-11.
Results
From overall 125 ICD-10 entities spread in 6 chapters, 57.6% were able to be precoordinated, 4% postcoordinated, 12% indexed to the Foundation, 9.6% had no code fitting properly and 18.6% had no correspondence in the ICD-11 framework.
Conclusion
We have been able to demonstrate that 83.2% of the ICD-10 allergic and hypersensitivity conditions could be captured by the current ICD-11 beta draft framework. We strongly believe that our findings constitute a key step forward for a softer transition of the ICD-10 allergic and hypersensitivity conditions to the ICD-11, supporting the WHO in this process as well as strengthening the visibility of the Allergy specialty and ensuring quality management of allergic patients.
The International Classification of Diseases (ICD) is a system of classifying and coding medical diagnosis maintained and revised by the World Health Organization (WHO) [1]. It is used as a worldwide tool for morbidity and mortality statistics, reimbursement systems, and automated decision support in health care. However, the ICD frame has never prioritized allergic and hypersensitivity conditions morbidity [2] and mortality data [3].
The ICD is revised periodically and most of the countries are currently operating with the 10th version (ICD-10). Work on ICD-10 began in 1983, and was made available in 1990, but some countries, such as Australia, Canada and United States of America, have been using national adaptations of the ICD-10.
The WHO started to work in the 11th revision of the ICD in 2011 and the presentation of the final version to World Health Assembly endorsement is scheduled to 2018 [1]. The ICD-11 revision was set in to update the framework with the new knowledge generated since the last revision and proposes a different philosophy from the previous ICD editions. For the first time, the WHO opened the online ICD revision for public discussion in order to strengthen awareness and acceptability worldwide, making it more feasible to end-users. The multihierarchy scheme is based on a Foundation, where it is possible to reach each entity, and a Linearization, containing the parents and stem codes. The ICD-11 prioritizes the postcoordination, defined by the adding to an existing entity, known as a stem code, additional details to provide greater specificity to the entity, such as topography, laterality, chronological and severity scale, now available in the ICD-11 "Extension codes" chapter. Although the ICD-11 is still a way from being officially ready for use, the WHO will advise all the countries to move to ICD-11 when it will made available.
In order to create a more appropriate classification for allergic and hypersensitivity conditions in the ICD-11, a detailed action plan was coordinated based on scientific evidences for the necessity of changes [234567891011]. Collaboration with the WHO ICD-11 revision governance is ongoing and all the efforts are being acknowledged by the Joint Allergy Academies, composed by the American Academy of Allergy Asthma and Immunology, the European Academy of Allergy and Clinical Immunology, the World Allergy Organization, the American College of Allergy Asthma and Immunology, the Asia Pacific Association of Allergy, Asthma and Clinical Immunology, and the Latin American Society of Allergy, Asthma and Immunology. The main achievement of this process was the construction of the "Allergic and hypersensitivity conditions" section in the ICD-11 beta draft under the chapter "Disorders of the immune system" [812]. The new frame has been built with full agreement of the involved Topic Advisory Groups and Expert Working Groups. Currently, the ICD-11 "Allergic and hypersensitivity conditions" section is composed by 6 mains headings (Fig. 1).
Considering the new classification model addressed to the allergic and hypersensitivity conditions and following ICD WHO agenda, we believe it is the appropriate time to start supporting the validation process in collaboration with the WHO ICD governance. The WHO states the mapping procedure as a validation tool to ensure stability of the new frame. Therefore, we conducted a mapping of ICD-10 allergic and hypersensitivity conditions in the ICD-11 beta phase structure.
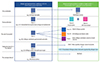
With the aim of identifying the allergic and hypersensitivity conditions scattered in the ICD-10, we first conducted a search for all the relevant related terms in the online ICD-10 2016 version [13]. In this process, we included all child codes (with more details) listed under a parent allergy code. We decided for not refer terms used to label family history or isolated symptoms (e.g., cough) to categorizing allergic and hypersensitivity conditions because these did not relate to the person or condition themselves. The prepared list and the online ICD-11 beta draft (April 2016 version) [12] were the basis of the manual mapping procedure. We carefully looked for the corresponding allergic and hypersensitivity conditions terms (Supplement material) in ICD-11 beta draft and categorized them as fitting by "precoordination," "postcoordination," "indexed to the ICD-11 Foundation," "no code fit properly" or "no correspondence" in the ICD-11 (Fig. 2). Two reviewers were involved independently in the ICD-10 selection terms process and mapping; disagreements were resolved by discussion. Conceptually, "precoordination" is applied in cases the conditions have direct correspondence (e.g., L20 Atopic dermatitis of ICD-10 = FB20 Atopic eczema of ICD-11). "Postcoordination" is the adding to a stem term additional characterization or specification (e.g., L27.0 Generalized skin eruption due to drugs and medicaments of ICD10 = drug eruption heading + XB13 Generalized of ICD-11). The innovatory ICD-11 revision assumed new concepts and adopted the digital format to support the complex multihierarchy structure. One of the most relevant implementations was adding the Foundation into the online platform. The Foundation contains everything in ICD and can be understood as a digital library including all ICD entities and is linked to the Linearization by a link, called Index. This method has been created to capture all the conditions, but, in general, the terms that are "indexed" are those with lower prevalence. Thus, we classified as "Indexed to the ICD-11 Foundation," all the terms absent in the Linearization, but available into the Foundation by Index (e.g., L23.4 Allergic contact dermatitis due to dyes of ICD-10 = Allergic contact dermatitis organized by allergen class of ICD-11 Foundation). Conditions labeled as "no code fit properly" where those possible to only partly capture the ICD-10 (e.g., K52.2 Allergic and dietetic gastroenteritis and colitis [food hypersensitivity gastroenteritis or colitis] of ICD-10 = EC60.31 Allergic gastritis, EC91.31 Allergic duodenitis, ED79 Allergic and dietetic colitis, ED24.3 Allergic and dietetic enteritis of small intestine of ICD-11). If the above strategy failed in identifying the corresponding condition, this was considered as "no correspondence."
In order to better understand the reasons why some conditions did not reach correspondences, we carefully reviewed each of them and classified these conditions as "classification change," "title change," and "not elsewhere classified (NEC)." ICD revision processes update classifications and incorporate new knowledge; therefore, some classifications may be abandoned. Thus, the "no correspondent" terms characterized as "classification change" covered all the terms not considered due to updated classification (e.g., L50.1 Idiopathic urticaria). In the same way, some titles have changes (e.g., L51 Erythema multiforme, used in ICD-10 as a title for severe bullous drug eruptions such as Stevens-Johnson syndrome and Toxic Epidermal Necrolysis).
The 125 terms implying allergic and hypersensitivity conditions in ICD-10 were scattered in 6 different chapters: "Diseases of the eye and adnexa" (11 terms), "Diseases of the respiratory system" (33 terms), "Diseases of the digestive system" (7 terms), "Diseases of the skin and subcutaneous tissue" (45 terms), "Injury, poisoning and certain other consequences of external causes" (13 terms), and "Factors influencing health status and contact with health services" (16 terms). The allergic and hypersensitivity conditions described under the "Injury, poisoning and certain other consequences of external causes" chapter were mainly related to anaphylaxis and its different causes, but still classified under the "T78 Adverse effects, not elsewhere classified."
By applying the mapping procedure, 57.6% were able to be precoordinated, 4% postcoordinated, 12% indexed to the Foundation, 9.6% had no code fitting properly and 14.4% had no correspondence in the ICD-11 framework (Table 1). The postcoordination strategy was mostly applicable to "Diseases of the respiratory system" and to "Diseases of the skin and subcutaneous tissue." On the other hand, the precoordination has been exclusively applied to "Diseases of the eye and adnexa" and "Diseases of the skin and subcutaneous tissue." There were no disagreements between the independent evaluators.
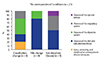
From overall 21 conditions (16.8%) categorized as "no correspondence," most were due to "classification changes" (38%) and to "title changes" (38%). "Title changes" and "NEC" terms were mostly observed in the "Diseases of the skin and subcutaneous tissue," but the "classification changes" were the main cause of no correspondence of "Diseases of the digestive system" in ICD-11 (Fig. 3).
The proposed document is the first attempt to substantiate the validation of the "Allergic and hypersensitivity conditions" new section of the ICD-11, by applying the mapping methodology. The mapping procedure has been in use to evaluate the stability of a new classification system. From the WHO perspective, it is an essential tool to ensure conceptual and structural transition process to the ICD-11. We have been able to demonstrate that 83.2% of the ICD-10 allergic and hypersensitivity conditions could be captured by the current ICD-11 beta draft framework. Substantial and expected updates in the classification and terminology of the disorders are responsible for the remaining 16.8%, but most of the terms may be linked to avoid losses. In fact, the new "Allergic and hypersensitivity conditions" parented chapter tried to cover most of previously highlighted gaps and trade-offs [2], by incorporating the principle of an allergy section into the ICD-11 beta draft. This model is the end result of the classification proposal coming from consensus academic reports [141516171819202122232425262728293031323334353637383940414243] and previously validated by crowdsourcing the allergists' community [5], the discussion with WHO ICD governance and organ specialists advisory boards of the WHO. This process has been constructed on a solid collaborative academic basis, incorporating the discussions with the WHO ICD representatives. By consolidating all allergic conditions into one ICD-11 single section, all the relevant codes will be able to be used to represent morbidity and mortality outcomes and to facilitate the use of such classification and codes by all relevant personnel. Although this manuscript presented some technical aspects of the validation process, we intended to introduce them to make them familiar to the allergy community ICD end-users, preparing them to the new coding procedures, whenever it is available.
The transition from ICD-10 to ICD-11 promises to produce an enhanced classification that will have better potential to capture important concepts relevant to measuring safety and quality management care. The built ICD-10 to ICD-11 bridge from the allergic and hypersensitivity conditions perspective allowed us to understand the remaining areas requiring implementation and was the basis for further discussion with the WHO governance during a WHO face-to-face meeting, which took place in Geneva, May 12th and 13th 2016. During this 2-day meeting, besides the here presented results and the outcomes of the survey of the new model [44], we could discuss the technical ways to ensure quality, feasibility and stability of the new frame.
The limitations of the current study have to be considered. The ICD-11 beta draft platform is regularly updated. Although the current WHO ICD revision representatives point out that this framework is not intended to be substantially modified, there is a risk of having the classification tuned up until the end of the revision process. That is a possibility that we cannot foresee on the manual classification, although we tried to minimize it by performing classification by independent investigators.
The presented results were able to draft the first map and track the ICD-10 to ICD-11 bridge for allergic and hypersensitivity conditions. We strongly believe that our findings constitute a key step forward for a softer transition of the ICD-10 allergic and hypersensitivity conditions to the ICD-11, supporting the WHO in this process as well as strengthening the visibility of the Allergy specialty and ensuring quality management of allergic patients.
Figures and Tables
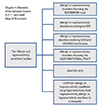 | Fig. 1The headings of the new "Allergic and hypersensitivity conditions" section of the "Disorders of the Immune System (ICD-11 beta draft, May 2016 version)". ICD, International Classification of Diseases. |
ACKNOWLEDGEMENTS
Luciana Kase Tanno received a grant from the Brazilian National Council for Scientific and Technological Development (CNPq).
We are extremely grateful to all the representatives of the ICD-11 revision with whom we have been carrying on fruitful discussions, helping us to tune the here presented classification: Robert Jakob, Linda Best, Nenad Kostanjsek, Robert J G Chalmers, Jeffrey Linzer, Linda Edwards, Ségolène Ayme, Bertrand Bellet, Rodney Franklin, Matthew Helbert, August Colenbrander, Satoshi Kashii, Paulo E. C. Dantas, Christine Graham, Ashley Behrens, Julie Rust, Megan Cumerlato, Tsutomu Suzuki, Mitsuko Kondo, Hajime Takizawa, Nobuoki Kohno, Soichiro Miura, Nan Tajima and Toshio Ogawa.
References
1. World Health Organization. International Classification of Diseases [Internet]. Geneva: World Health Organization;c2016. cited 2016 Jun 1. Available from: http://www.who.int/classifications/icd/en.
2. Tanno LK, Calderon MA, Goldberg BJ, Akdis CA, Papadopoulos NG, Demoly P. Categorization of allergic disorders in the new World Health Organization International Classification of Diseases. Clin Transl Allergy. 2014; 4:42.

3. Tanno LK, Ganem F, Demoly P, Toscano CM, Bierrenbach AL. Undernotification of anaphylaxis deaths in Brazil due to difficult coding under the ICD-10. Allergy. 2012; 67:783–789.

4. Demoly P, Tanno LK, Akdis CA, Lau S, Calderon MA, Santos AF, Sanchez-Borges M, Rosenwasser LJ, Pawankar R, Papadopoulos NG. Global classification and coding of hypersensitivity diseases - An EAACI - WAO survey, strategic paper and review. Allergy. 2014; 69:559–570.

5. Tanno LK, Calderon MA, Goldberg BJ, Gayraud J, Bircher AJ, Casale T, Li J, Sanchez-Borges M, Rosenwasser LJ, Pawankar R, Papadopoulos NG, Demoly P. Constructing a classification of hypersensitivity/allergic diseases for ICD-11 by crowdsourcing the allergist community. Allergy. 2015; 70:609–615.

6. Tanno LK, Calderon M, Papadopoulos NG, Demoly P. EAACI/WAO Task force of a Global Classification of Hypersensitivity/Allergic diseases. Mapping hypersensitivity/allergic diseases in the International Classification of Diseases (ICD)-11: cross-linking terms and unmet needs. Clin Transl Allergy. 2015; 5:20.

7. Tanno LK, Calderon MA, Demoly P. Allergy Academies. Optimization and simplification of the Allergic and Hypersensitivity conditions classification for the ICD-11. Allergy. 2016; 71:671–676.

8. Tanno LK, Calderon MA, Demoly P. Joint Allergy Academies. New allergic and hypersensitivity conditions section in the International Classification of Diseases-11. Allergy Asthma Immunol Res. 2016; 8:383–388.

9. Tanno LK, Calderon MA, Demoly P. Making allergic and hypersensitivity conditions visible in the International Classification of Diseases-11. Asia Pac Allergy. 2015; 5:193–196.

10. Tanno LK, Calderon MA, Li J, Casale T, Demoly P. Joint Allergy Academies. Updating allergy and/or hypersensitivity diagnostic procedures in the WHO ICD-11 revision. J Allergy Clin Immunol Pract. 2016; 4:650–657.

11. Tanno LK, Calderon MA, Papadopoulos NG, Sanchez-Borges M, Rosenwasser LJ, Bousquet J, Pawankar R, Sisul JC, Cepeda AM, Li J, Muraro A, Fineman S, Sublett JL, Katelaris CH, Chang YS, Moon HB, Casale T, Demoly P. Joint Allergy Academies. Revisiting desensitization and allergen immunotherapy concepts for the International Classification of Diseases (ICD)-11. J Allergy Clin Immunol Pract. 2016; 4:643–649.

12. World Health Organization. ICD-11 beta draft [Internet]. Geneva: World Health Organization;c2016. cited 2016 Jun 1. Available from:http://apps.who.int/classifications/icd11/browse/l-m/en.
13. World Health Organization. ICD-10 version 2016 [Internet]. Geneva: World Health Organization;c2016. cited 2016 Jun 1. Available from: http://apps.who.int/classifications/icd10/browse/2016/en.
14. Johansson SG, Bieber T, Dahl R, Friedmann PS, Lanier BQ, Lockey RF, Motala C, Ortega Martell JA, Platts-Mills TA, Ring J, Thien F, Van Cauwenberge P, Williams HC. Revised nomenclature for allergy for global use: Report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. J Allergy Clin Immunol. 2004; 113:832–836.

15. Sampson HA, Muñoz-Furlong A, Campbell RL, Adkinson NF Jr, Bock SA, Branum A, Brown SG, Camargo CA Jr, Cydulka R, Galli SJ, Gidudu J, Gruchalla RS, Harlor AD Jr, Hepner DL, Lewis LM, Lieberman PL, Metcalfe DD, O'Connor R, Muraro A, Rudman A, Schmitt C, Scherrer D, Simons FE, Thomas S, Wood JP, Decker WW. Second symposium on the definition and management of anaphylaxis: summary report--Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol. 2006; 117:391–397.

16. Scadding G, Hellings P, Alobid I, Bachert C, Fokkens W, van Wijk RG, Gevaert P, Guilemany J, Kalogjera L, Lund V, Mullol J, Passalacqua G, Toskala E, van Drunen C. Diagnostic tools in Rhinology EAACI position paper. Clin Transl Allergy. 2011; 1:2.

17. Fokkens W, Lund V, Mullol J. European Position Paper on Rhinosinusitis and Nasal Polyps group. European position paper on rhinosinusitis and nasal polyps 2007. Rhinol Suppl. 2007; (20):1–136.
18. Bousquet J, Schunemann HJ, Zuberbier T, Bachert C, Baena-Cagnani CE, Bousquet PJ, Brozek J, Canonica GW, Casale TB, Demoly P, Gerth van Wijk R, Ohta K, Bateman ED, Calderon M, Cruz AA, Dolen WK, Haughney J, Lockey RF, Lötvall J, O'Byrne P, Spranger O, Togias A, Bonini S, Boulet LP, Camargos P, Carlsen KH, Chavannes NH, Delgado L, Durham SR, Fokkens WJ, Fonseca J, Haahtela T, Kalayci O, Kowalski ML, Larenas-Linnemann D, Li J, Mohammad Y, Mullol J, Naclerio R, O'Hehir RE, Papadopoulos N, Passalacqua G, Rabe KF, Pawankar R, Ryan D, Samolinski B, Simons FE, Valovirta E, Yorgancioglu A, Yusuf OM, Agache I, Aït-Khaled N, Annesi-Maesano I, Beghe B, Ben Kheder A, Blaiss MS, Boakye DA, Bouchard J, Burney PG, Busse WW, Chan-Yeung M, Chen Y, Chuchalin AG, Costa DJ, Custovic A, Dahl R, Denburg J, Douagui H, Emuzyte R, Grouse L, Humbert M, Jackson C, Johnston SL, Kaliner MA, Keith PK, Kim YY, Klossek JM, Kuna P, Le LT, Lemiere C, Lipworth B, Mahboub B, Malo JL, Marshall GD, Mavale-Manuel S, Meltzer EO, Morais-Almeida M, Motala C, Naspitz C, Nekam K, Niggemann B, Nizankowska-Mogilnicka E, Okamoto Y, Orru MP, Ouedraogo S, Palkonen S, Popov TA, Price D, Rosado-Pinto J, Scadding GK, Sooronbaev TM, Stoloff SW, Toskala E, van Cauwenberge P, Vandenplas O, van Weel C, Viegi G, Virchow JC, Wang DY, Wickman M, Williams D, Yawn BP, Zar HJ, Zernotti M, Zhong N. WHO Collaborating Center of Asthma and Rhinitis (Montpellier). Development and implementation of guidelines in allergic rhinitis – an ARIA-GA2LEN paper. Allergy. 2010; 65:1212–1221.

19. Bacharier LB, Boner A, Carlsen KH, Eigenmann PA, Frischer T, Gotz M, Helms PJ, Hunt J, Liu A, Papadopoulos N, Platts-Mills T, Pohunek P, Simons FE, Valovirta E, Wahn U, Wildhaber J. European Pediatric Asthma Group. Diagnosis and treatment of asthma in childhood: a PRACTALL consensus report. Allergy. 2008; 63:5–34.

20. World Health Organization. Chronic respiratory disease: asthma [Internet]. Geneva: World Health Organization;c2016. cited 2016 Jun 1. Available from: http://www.who.int/respiratory/asthma/en/.
21. Masoli M, Fabian D, Holt S, Beasley R. Global Initiative for Asthma (GINA) Program. GINA) Program. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy. 2004; 59:469–478.
22. Bateman ED, Bousquet J, Keech ML, Busse WW, Clark TJ, Pedersen SE. The correlation between asthma control and health status: the GOAL study. Eur Respir J. 2007; 29:56–62.

23. Papadopoulos NG, Arakawa H, Carlsen KH, Custovic A, Gern J, Lemanske R, Le Souef P, Mäkelä M, Roberts G, Wong G, Zar H, Akdis CA, Bacharier LB, Baraldi E, van Bever HP, de Blic J, Boner A, Burks W, Casale TB, Castro-Rodriguez JA, Chen YZ, El-Gamal YM, Everard ML, Frischer T, Geller M, Gereda J, Goh DY, Guilbert TW, Hedlin G, Heymann PW, Hong SJ, Hossny EM, Huang JL, Jackson DJ, de Jongste JC, Kalayci O, Aït-Khaled N, Kling S, Kuna P, Lau S, Ledford DK, Lee SI, Liu AH, Lockey RF, Lødrup-Carlsen K, Lötvall J, Morikawa A, Nieto A, Paramesh H, Pawankar R, Pohunek P, Pongracic J, Price D, Robertson C, Rosario N, Rossenwasser LJ, Sly PD, Stein R, Stick S, Szefler S, Taussig LM, Valovirta E, Vichyanond P, Wallace D, Weinberg E, Wennergren G, Wildhaber J, Zeiger RS. International consensus on (ICON) pediatric asthma. Allergy. 2012; 67:976–997.

24. Burks AW, Tang M, Sicherer S, Muraro A, Eigenmann PA, Ebisawa M, Fiocchi A, Chiang W, Beyer K, Wood R, Hourihane J, Jones SM, Lack G, Sampson HA. ICON: food allergy. J Allergy Clin Immunol. 2012; 129:906–920.

25. Simons FE, Ardusso LR, Bilo MB, Cardona V, Ebisawa M, El-Gamal YM, Lieberman P, Lockey RF, Muraro A, Roberts G, Sanchez-Borges M, Sheikh A, Shek LP, Wallace DV, Worm M. International consensus on (ICON) anaphylaxis. World Allergy Organ J. 2014; 7:9.

26. Moscato G, Pala G, Cullinan P, Folletti I, Gerth van Wijk R, Pignatti P, Quirce S, Sastre J, Toskala E, Vandenplas O, Walusiak-Skorupa J, Malo JL. EAACI Position Paper on assessment of cough in the workplace. Allergy. 2014; 69:292–304.

27. Leonardi A, Bogacka E, Fauquert JL, Kowalski ML, Groblewska A, Jedrzejczak-Czechowicz M, Doan S, Marmouz F, Demoly P, Delgado L. Ocular allergy: recognizing and diagnosing hypersensitivity disorders of the ocular surface. Allergy. 2012; 67:1327–1337.

28. La Rosa M, Lionetti E, Reibaldi M, Russo A, Longo A, Leonardi S, Tomarchio S, Avitabile T, Reibaldi A. Allergic conjunctivitis: a comprehensive review of the literature. Ital J Pediatr. 2013; 39:18.

29. Bonini S, Coassin M, Aronni S, Lambiase A. Vernal keratoconjunctivitis. Eye (Lond). 2004; 18:345–351.

30. Akdis CA, Akdis M, Bieber T, Bindslev-Jensen C, Boguniewicz M, Eigenmann P, Hamid Q, Kapp A, Leung DY, Lipozencic J, Luger TA, Muraro A, Novak N, Platts-Mills TA, Rosenwasser L, Scheynius A, Simons FE, Spergel J, Turjanmaa K, Wahn U, Weidinger S, Werfel T, Zuberbier T. European Academy of Allergology. Clinical Immunology/American Academy of Allergy, Asthma and Immunology/PRACTALL Consensus Group. Diagnosis and treatment of atopic dermatitis in children and adults: European Academy of Allergology and Clinical Immunology/American Academy of Allergy, Asthma and Immunology/PRACTALL Consensus Report. Allergy. 2006; 61:969–987.

31. Bonitsis NG, Tatsioni A, Bassioukas K, Ioannidis JP. Allergens responsible for allergic contact dermatitis among children: a systematic review and meta-analysis. Contact Dermatitis. 2011; 64:245–257.

32. Tan CH, Rasool S, Johnston GA. Contact dermatitis: allergic and irritant. Clin Dermatol. 2014; 32:116–124.

33. Zuberbier T, Asero R, Bindslev-Jensen C, Walter Canonica G, Church MK, Gimenez-Arnau A, Grattan CE, Kapp A, Merk HF, Rogala B, Saini S, Sánchez-Borges M, Schmid-Grendelmeier P, Schunemann H, Staubach P, Vena GA, Wedi B, Maurer M. Dermatology Section of the European Academy of Allergology and Clinical Immunology. Global Allergy and Asthma European Network. European Dermatology Forum. World Allergy Organization. EAACI/GA(2)LEN/EDF/WAO guideline: definition, classification and diagnosis of urticaria. Allergy. 2009; 64:1417–1426.
34. Cicardi M, Bork K, Caballero T, Craig T, Li HH, Longhurst H, Reshef A, Zuraw B. HAWK (Hereditary Angioedema International Working Group). Evidence-based recommendations for the therapeutic management of angioedema owing to hereditary C1 inhibitor deficiency: consensus report of an International Working Group. Allergy. 2012; 67:147–157.

35. Craig T, Aygoren-Pursun E, Bork K, Bowen T, Boysen H, Farkas H, Grumach A, Katelaris CH, Lockey R, Longhurst H, Lumry W, Magerl M, Martinez-Saguer I, Ritchie B, Nast A, Pawankar R, Zuraw B, Maurer M. WAO guideline for the management of hereditary angioedema. World Allergy Organ J. 2012; 5:182–199.

36. Soares-Weiser K, Panesar SS, Rader T, Takwoingi Y, Werfel T, Muraro A, Hoffmann-Sommergruber K, Roberts G, Sheikh A. EAACI Food Allergy and Anaphylaxis Group. The diagnosis of food allergy: protocol for a systematic review. Clin Transl Allergy. 2013; 3:18.
37. Bircher AJ, Scherer K. Delayed cutaneous manifestations of drug hypersensitivity. Med Clin North Am. 2010; 94:711–725.

38. Pirmohamed M, Friedmann PS, Molokhia M, Loke YK, Smith C, Phillips E, La Grenade L, Carleton B, Papaluca-Amati M, Demoly P, Shear NH. Phenotype standardization for immune-mediated drug-induced skin injury. Clin Pharmacol Ther. 2011; 89:896–901.

40. Bilo BM, Rueff F, Mosbech H, Bonifazi F, Oude-Elberink JN. EAACI Interest Group on Insect Venom Hypersensitivity. Diagnosis of hymenoptera venom allergy. Allergy. 2005; 60:1339–1349.

41. Bonifazi F, Jutel M, Bilo BM, Birnbaum J, Muller U. EAACI Interest Group on Insect Venom Hypersensitivity. Prevention and treatment of hymenoptera venom allergy: guidelines for clinical practice. Allergy. 2005; 60:1459–1470.

42. EAACI food allergy and anaphylaxis guidelines [Internet]. Zurich (CH): European Academy of Allergy and Clinical Immunology;2016. cited 2016 Jun 1. Available from: http://www.eaaci.org/resources/scientific-output/guidelines/2533-food-allergy-and-anaphylaxis-guideline.html.
43. Simons FE, Ebisawa M, Sanchez-Borges M, Thong BY, Worm M, Tanno LK, Lockey RF, El-Gamal YM, Brown SG, Park HS, Sheikh A. 2015 update of the evidence base: World Allergy Organization anaphylaxis guidelines. World Allergy Organ J. 2015; 8:32.

44. Tanno LK, Calderon MA, Papadopoulos NG, Sanchez-Borges M, Moon HB, Sisul JC, Jares EJ, Sublett JL, Casale T, Demoly P. Joint Allergy Academies. Surveying the new allergic and hypersensitivity conditions chapter of the International classification of diseases (ICD)-11. Allergy. 2016; 06. 02. [Epub]. DOI: 10.1111/all.12945.

SUPPLEMENTARY MATERIAL
Supplementary material can be found via http://www.apallergy.org/src/sm/apallergy-6-149-s001.xlsx.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download